当前位置:
X-MOL 学术
›
Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
GDOES Analysis of Niobium De-hydrogenation after Electropolishing Processes
Materials Letters ( IF 2.7 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.matlet.2018.02.027 Tadeusz Hryniewicz , Krzysztof Rokosz , Sofia Gaiaschi , Patrick Chapon , Ryszard Rokicki , Dalibor Matysek
Materials Letters ( IF 2.7 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.matlet.2018.02.027 Tadeusz Hryniewicz , Krzysztof Rokosz , Sofia Gaiaschi , Patrick Chapon , Ryszard Rokicki , Dalibor Matysek
|
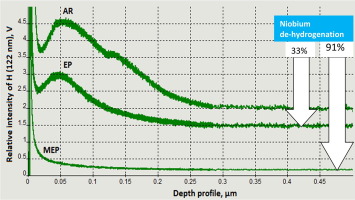
Abstract Niobium, as pure metal and alloying element, is used in a variety of applications, among them in nuclear industries. Niobium is incorporated into nuclear fission reactors due to its enormous strength and relatively low density. Surface finishing of niobium is often performed in electrochemical polishing processes in view of improving its smoothness, corrosion resistance and its surface cleanability. However, the presently used electropolishing process (EP) is intrinsically linked to the subsurface hydrogenation of niobium, which measurably degrades its properties. The annealing operation, which is used to remove hydrogen from electropolished niobium, is quite a costly and time-consuming process. The traditional electrolyte consisting of a mixture of 96% H2SO4/49% HF acids by volume in a 9:1 ratio has been substituted for the new one, being a mixture of 70% methanesulfonic acid with 49% hydrofluoric acid by volume in a 3:1 ratio. The additional imposition of a magnetic field during the electropolishing process – magnetoelectropolishing (MEP) further increases hydrogen removal, when compared to the hydrogen content achieved by the electropolishing process (EP) alone. The aim of the study is to reveal a methodic approach and showing decreasing hydrogenation of niobium samples after consecutive steps of electrochemical polishing. Glow-Discharge Optical Emission Spectroscopy (GDOES) measurements were used to measure the hydrogen content in the surface layer of as-received (AR) niobium and in the samples after EP and MEP processes, and prove its close-to-zero content after MEP.
中文翻译:

电解抛光后铌脱氢的 GDOES 分析
摘要 铌作为纯金属和合金元素,用途广泛,其中包括核工业。铌因其巨大的强度和相对较低的密度而被纳入核裂变反应堆。铌的表面精加工通常在电化学抛光过程中进行,以提高其光滑度、耐腐蚀性和表面清洁性。然而,目前使用的电解抛光工艺 (EP) 与铌的表面下氢化有内在联系,这会显着降低其性能。用于从电解抛光的铌中去除氢的退火操作是一个相当昂贵且耗时的过程。由体积比为 9:1 的 96% H2SO4/49% HF 酸混合物组成的传统电解液已被新电解液取代,70% 的甲磺酸和 49% 的氢氟酸以 3:1 的体积比混合。与单独通过电解抛光工艺 (EP) 实现的氢含量相比,在电解抛光工艺期间额外施加的磁场 - 磁电抛光 (MEP) 进一步提高了氢去除率。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与单独通过电解抛光工艺 (EP) 实现的氢含量相比,在电解抛光工艺期间额外施加的磁场 - 磁电抛光 (MEP) 进一步提高了氢去除率。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与单独通过电解抛光工艺 (EP) 实现的氢含量相比,在电解抛光工艺期间额外施加的磁场 - 磁电抛光 (MEP) 进一步提高了氢去除率。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与仅通过电解抛光工艺 (EP) 获得的氢含量相比。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与仅通过电解抛光工艺 (EP) 获得的氢含量相比。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零.
更新日期:2018-05-01
中文翻译:

电解抛光后铌脱氢的 GDOES 分析
摘要 铌作为纯金属和合金元素,用途广泛,其中包括核工业。铌因其巨大的强度和相对较低的密度而被纳入核裂变反应堆。铌的表面精加工通常在电化学抛光过程中进行,以提高其光滑度、耐腐蚀性和表面清洁性。然而,目前使用的电解抛光工艺 (EP) 与铌的表面下氢化有内在联系,这会显着降低其性能。用于从电解抛光的铌中去除氢的退火操作是一个相当昂贵且耗时的过程。由体积比为 9:1 的 96% H2SO4/49% HF 酸混合物组成的传统电解液已被新电解液取代,70% 的甲磺酸和 49% 的氢氟酸以 3:1 的体积比混合。与单独通过电解抛光工艺 (EP) 实现的氢含量相比,在电解抛光工艺期间额外施加的磁场 - 磁电抛光 (MEP) 进一步提高了氢去除率。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与单独通过电解抛光工艺 (EP) 实现的氢含量相比,在电解抛光工艺期间额外施加的磁场 - 磁电抛光 (MEP) 进一步提高了氢去除率。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与单独通过电解抛光工艺 (EP) 实现的氢含量相比,在电解抛光工艺期间额外施加的磁场 - 磁电抛光 (MEP) 进一步提高了氢去除率。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与仅通过电解抛光工艺 (EP) 获得的氢含量相比。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零. 与仅通过电解抛光工艺 (EP) 获得的氢含量相比。该研究的目的是揭示一种有条理的方法,并显示在连续的电化学抛光步骤后铌样品的氢化减少。辉光放电发射光谱 (GDOES) 测量用于测量原样 (AR) 铌表面层和 EP 和 MEP 工艺后样品中的氢含量,并证明其在 MEP 后含量接近于零.











































 京公网安备 11010802027423号
京公网安备 11010802027423号