当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The emerging era of supramolecular polymeric binders in silicon anodes
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1039/c7cs00858a Tae-woo Kwon 1, 2, 3, 4, 5 , Jang Wook Choi 6, 7, 8, 9 , Ali Coskun 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1039/c7cs00858a Tae-woo Kwon 1, 2, 3, 4, 5 , Jang Wook Choi 6, 7, 8, 9 , Ali Coskun 1, 2, 3, 4, 5
Affiliation
|
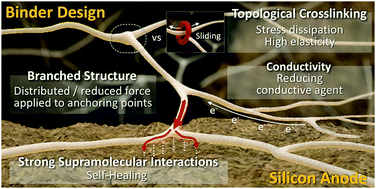
Silicon (Si) anode is among the most promising candidates for the next-generation high-capacity electrodes in Li-ion batteries owing to its unparalleled theoretical capacity (4200 mA h g−1 for Li4.4Si) that is approximately 10 times higher than that of commercialized graphitic anodes (372 mA h g−1 for LiC6). The battery community has witnessed substantial advances in research on new polymeric binders for silicon anodes mainly due to the shortcomings of conventional binders such as polyvinylidene difluoride (PVDF) to address problems caused by the massive volume change of Si (300%) upon (de)lithiation. Unlike conventional battery electrodes, polymeric binders have been shown to play an active role in silicon anodes to alleviate various capacity decay pathways. While the initial focus in binder research was primarily to maintain the electrode morphology, it has been recently shown that polymeric binders can in fact help to stabilize cracked Si microparticles along with the solid–electrolyte-interphase (SEI) layer, thus substantially improving the electrochemical performance. In this review article, we aim to provide an in-depth analysis and molecular-level design principles of polymeric binders for silicon anodes in terms of their chemical structure, superstructure, and supramolecular interactions to achieve good electrochemical performance. We further highlight that supramolecular chemistry offers practical tools to address challenging problems associated with emerging electrode materials in rechargeable batteries.
中文翻译:

硅阳极中超分子聚合物粘合剂的新兴时代
硅(Si)阳极由于其无与伦比的理论容量(Li 4.4 Si为4200 mA hg -1),比锂离子电池高约10倍,因此是锂离子电池下一代高容量电极最有希望的候选者之一商业化的石墨阳极(对于LiC 6为372 mA hg -1)。电池界已经见证了用于硅阳极的新型聚合物粘合剂的研究取得了重大进展,这主要是由于常规粘合剂(如聚偏二氟乙烯(PVDF))的缺点所致,该缺陷解决了(de)时硅(300%)的大量体积变化所引起的问题。锂化。与常规电池电极不同,聚合物粘合剂已显示出在硅阳极中发挥积极作用,以减轻各种容量衰减途径。尽管粘合剂研究的最初重点主要是保持电极的形态,但最近发现,聚合物粘合剂实际上可以帮助稳定破裂的Si微粒以及固体-电解质中间相(SEI)层,从而显着改善电化学性能。表现。在这篇评论文章中,我们旨在针对硅阳极的聚合物粘合剂的化学结构,超结构和超分子相互作用提供深入的分析和分子水平的设计原理,以实现良好的电化学性能。我们进一步强调,超分子化学提供了实用的工具来解决与可充电电池中新兴的电极材料相关的挑战性问题。
更新日期:2018-02-07
中文翻译:

硅阳极中超分子聚合物粘合剂的新兴时代
硅(Si)阳极由于其无与伦比的理论容量(Li 4.4 Si为4200 mA hg -1),比锂离子电池高约10倍,因此是锂离子电池下一代高容量电极最有希望的候选者之一商业化的石墨阳极(对于LiC 6为372 mA hg -1)。电池界已经见证了用于硅阳极的新型聚合物粘合剂的研究取得了重大进展,这主要是由于常规粘合剂(如聚偏二氟乙烯(PVDF))的缺点所致,该缺陷解决了(de)时硅(300%)的大量体积变化所引起的问题。锂化。与常规电池电极不同,聚合物粘合剂已显示出在硅阳极中发挥积极作用,以减轻各种容量衰减途径。尽管粘合剂研究的最初重点主要是保持电极的形态,但最近发现,聚合物粘合剂实际上可以帮助稳定破裂的Si微粒以及固体-电解质中间相(SEI)层,从而显着改善电化学性能。表现。在这篇评论文章中,我们旨在针对硅阳极的聚合物粘合剂的化学结构,超结构和超分子相互作用提供深入的分析和分子水平的设计原理,以实现良好的电化学性能。我们进一步强调,超分子化学提供了实用的工具来解决与可充电电池中新兴的电极材料相关的挑战性问题。









































 京公网安备 11010802027423号
京公网安备 11010802027423号