当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dinuclear Pathways for the Activation of Strained Three-Membered Rings
Organometallics ( IF 2.5 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1021/acs.organomet.7b00862 Heather R. Rounds 1 , Matthias Zeller 1 , Christopher Uyeda 1
Organometallics ( IF 2.5 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1021/acs.organomet.7b00862 Heather R. Rounds 1 , Matthias Zeller 1 , Christopher Uyeda 1
Affiliation
|
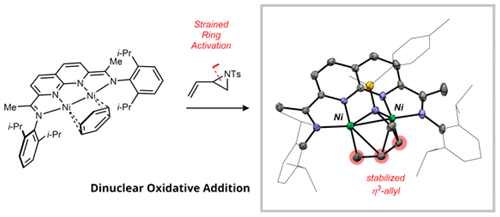
Dinuclear, strain-induced ring-opening reactions of vinylaziridines and vinylcyclopropanes are described. The previously reported [NDI]Ni2(C6H6) complex (NDI = naphthyridine–diimine) reacts with N-tosyl-2-vinylaziridine via C–N oxidative addition to generate a dinickel metallacyclic product. On the basis of this stoichiometric reactivity, the [NDI]Ni2(C6H6) complex is shown to be a highly active catalyst for the rearrangement of vinylcyclopropane to cyclopentene. Notably, 2-phenyl-1-vinylcyclopropane undergoes regioselective activation at the less hindered C–C bond in contrast to the noncatalytic thermal rearrangement. DFT calculations provide insight into the ability of the Ni–Ni bond to stabilize key intermediates and transition states along the catalytic pathway.
中文翻译:

激活三元环的双核途径
描述了乙烯基氮丙啶和乙烯基环丙烷的双核,应变诱导的开环反应。先前报道的[NDI] Ni 2(C 6 H 6)络合物(NDI =萘啶-二亚胺)通过C–N氧化加成与N-甲苯磺酰基-2-乙烯基氮丙啶反应生成二镍金属环产物。基于该化学计量反应性,[NDI] Ni 2(C 6 H 6)配合物被证明是将乙烯基环丙烷重排为环戊烯的高活性催化剂。值得注意的是,与非催化热重排相比,2-苯基-1-乙烯基环丙烷在受阻较小的C–C键处进行区域选择性活化。DFT计算提供了对Ni-Ni键沿催化途径稳定关键中间体和过渡态的能力的了解。
更新日期:2018-02-07
中文翻译:

激活三元环的双核途径
描述了乙烯基氮丙啶和乙烯基环丙烷的双核,应变诱导的开环反应。先前报道的[NDI] Ni 2(C 6 H 6)络合物(NDI =萘啶-二亚胺)通过C–N氧化加成与N-甲苯磺酰基-2-乙烯基氮丙啶反应生成二镍金属环产物。基于该化学计量反应性,[NDI] Ni 2(C 6 H 6)配合物被证明是将乙烯基环丙烷重排为环戊烯的高活性催化剂。值得注意的是,与非催化热重排相比,2-苯基-1-乙烯基环丙烷在受阻较小的C–C键处进行区域选择性活化。DFT计算提供了对Ni-Ni键沿催化途径稳定关键中间体和过渡态的能力的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号