当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conserved Histidine Adjacent to the Proximal Cluster Tunes the Anaerobic Reductive Activation of Escherichia coli Membrane‐Bound [NiFe] Hydrogenase‐1
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-16 , DOI: 10.1002/celc.201800047 Lindsey A Flanagan 1 , Harriet S Chidwick 1 , Julia Walton 1 , James W B Moir 2 , Alison Parkin 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-16 , DOI: 10.1002/celc.201800047 Lindsey A Flanagan 1 , Harriet S Chidwick 1 , Julia Walton 1 , James W B Moir 2 , Alison Parkin 1
Affiliation
|
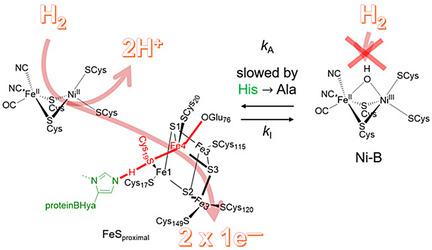
[NiFe] hydrogenases are electrocatalysts that oxidize H2 at a rapid rate without the need for precious metals. All membrane‐bound [NiFe] hydrogenases (MBH) possess a histidine residue that points to the electron‐transfer iron sulfur cluster closest (“proximal”) to the [NiFe] H2‐binding active site. Replacement of this amino acid with alanine induces O2 sensitivity, and this has been attributed to the role of the histidine in enabling the reversible O2‐induced over‐oxidation of the [Fe4S3Cys2] proximal cluster possessed by all O2‐tolerant MBH. We have created an Escherichia coli Hyd‐1 His‐to‐Ala variant and report O2‐free electrochemical measurements at high potential that indicate the histidine‐mediated [Fe4S3Cys2] cluster‐opening/closing mechanism also underpins anaerobic reactivation. We validate these experiments by comparing them to the impact of an analogous His‐to‐Ala replacement in Escherichia coli Hyd‐2, a [NiFe]‐MBH that contains a [Fe4S4] center.
中文翻译:

邻近近端簇的保守组氨酸调节大肠杆菌膜结合 [NiFe] 氢化酶-1 的厌氧还原激活
[NiFe]氢化酶是无需贵金属即可快速氧化H 2的电催化剂。所有膜结合的 [NiFe] 氢化酶 (MBH) 都具有组氨酸残基,该残基指向最接近(“最接近”)[NiFe] H 2结合活性位点的电子转移铁硫簇。用丙氨酸取代该氨基酸会诱导 O 2敏感性,这归因于组氨酸在使所有 O 所拥有的 [Fe 4 S 3 Cys 2 ] 近端簇发生可逆 O 2诱导的过度氧化中的作用。 2耐受MBH。我们创建了大肠杆菌Hyd-1 His-to-Ala 变体,并报告了高电位下的无 O 2电化学测量,表明组氨酸介导的 [Fe 4 S 3 Cys 2 ] 簇打开/关闭机制也支持厌氧再激活。我们通过将这些实验与大肠杆菌Hyd-2(一种包含 [Fe 4 S 4 ] 中心的 [NiFe]-MBH)中类似的 His-Ala 替代的影响进行比较来验证这些实验。
更新日期:2018-02-16
中文翻译:

邻近近端簇的保守组氨酸调节大肠杆菌膜结合 [NiFe] 氢化酶-1 的厌氧还原激活
[NiFe]氢化酶是无需贵金属即可快速氧化H 2的电催化剂。所有膜结合的 [NiFe] 氢化酶 (MBH) 都具有组氨酸残基,该残基指向最接近(“最接近”)[NiFe] H 2结合活性位点的电子转移铁硫簇。用丙氨酸取代该氨基酸会诱导 O 2敏感性,这归因于组氨酸在使所有 O 所拥有的 [Fe 4 S 3 Cys 2 ] 近端簇发生可逆 O 2诱导的过度氧化中的作用。 2耐受MBH。我们创建了大肠杆菌Hyd-1 His-to-Ala 变体,并报告了高电位下的无 O 2电化学测量,表明组氨酸介导的 [Fe 4 S 3 Cys 2 ] 簇打开/关闭机制也支持厌氧再激活。我们通过将这些实验与大肠杆菌Hyd-2(一种包含 [Fe 4 S 4 ] 中心的 [NiFe]-MBH)中类似的 His-Ala 替代的影响进行比较来验证这些实验。










































 京公网安备 11010802027423号
京公网安备 11010802027423号