当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioresponsive and near infrared photon co-enhanced cancer theranostic based on upconversion nanocapsules†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1039/c7sc05414a Jiating Xu 1 , Wei Han 1 , Ziyong Cheng 2 , Piaoping Yang 1 , Huiting Bi 1 , Dan Yang 1 , Na Niu 3 , Fei He 1 , Shili Gai 1 , Jun Lin 2
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1039/c7sc05414a Jiating Xu 1 , Wei Han 1 , Ziyong Cheng 2 , Piaoping Yang 1 , Huiting Bi 1 , Dan Yang 1 , Na Niu 3 , Fei He 1 , Shili Gai 1 , Jun Lin 2
Affiliation
|
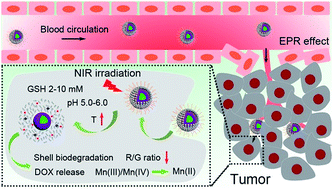
Developing nanotheranostics responsive to tumor microenvironments has attracted tremendous attention for on-demand cancer diagnosis and treatment. Herein, a facile Mn-doping strategy was adopted to transform mesoporous silica coated upconversion nanoparticles (UCNPs) to yolk-like upconversion nanostructures which possess a tumor-responsive biodegradation nature. The huge internal space of the innovated nanocarriers is suitable for doxorubicin (DOX) storage, besides, the Mn-doped shell is sensitive to the intratumoral acidity and reducibility, which enables shell biodegradation and further accelerates the breakage of Si–O–Si bonds within the silica framework. This tumor-responsive shell degradation is beneficial for realizing tumor-specific DOX release. Subsequently, polyoxometalate (POM) nanoclusters that can enhance photothermal conversion in response to the tumor reducibility and acidity were modified on the surface of the silica shell, thereby achieving NIR-enhanced shell degradation and also preventing premature DOX leakage. The as-produced thermal effect of the POM couples with the chemotherapy effect of the released DOX to perform a synergetic chemo-photothermal therapy. Additionally, the shell degradation brings size shrinkage to the nanocarriers, allowing faster nanoparticle diffusion and deeper tumor penetration, which is significant for improving theranostic outcomes. Also, the drastic decline of the red/green (R/G) ratio caused by the DOX release can be used to monitor the DOX release content through a fluorescence resonance energy transfer (FRET) method. The MRI effect caused by Mn release together with the MRI/CT/UCL imaging derived from Gd3+/Yb3+/Nd3+/Er3+ co-doped UCNPs under 808 nm laser excitation endow the nanosystem with multiple imaging capability, thus realizing imaging-guided cancer therapy.
中文翻译:

基于上转换纳米胶囊的生物响应和近红外光子共同增强癌症治疗诊断†
开发对肿瘤微环境敏感的纳米治疗学引起了按需癌症诊断和治疗的极大关注。在此,采用简单的锰掺杂策略将介孔二氧化硅涂覆的上转换纳米粒子(UCNP)转化为具有肿瘤响应性生物降解性质的蛋黄样上转换纳米结构。创新纳米载体巨大的内部空间适合阿霉素(DOX)的储存,此外,Mn掺杂的壳对瘤内酸性和还原性敏感,这使得壳能够生物降解,并进一步加速内部Si-O-Si键的断裂。二氧化硅框架。这种肿瘤响应性外壳降解有利于实现肿瘤特异性 DOX 释放。随后,在二氧化硅壳的表面修饰了多金属氧酸盐(POM)纳米团簇,该纳米团簇可以增强光热转换以响应肿瘤的还原性和酸性,从而实现近红外增强的壳降解并防止DOX过早泄漏。POM 产生的热效应与释放的 DOX 的化疗效应相结合,进行协同化学光热疗法。此外,外壳降解使纳米载体尺寸缩小,从而使纳米颗粒更快扩散和更深的肿瘤渗透,这对于改善治疗诊断结果具有重要意义。此外,DOX释放引起的红/绿(R/G)比值急剧下降可用于通过荧光共振能量转移(FRET)方法监测DOX释放含量。Mn释放引起的MRI效应以及Gd 3+ /Yb 3+ /Nd 3+ /Er 3+共掺杂UCNPs在808 nm激光激发下产生的MRI/CT/UCL成像赋予纳米系统多种成像能力,从而实现影像引导的癌症治疗。
更新日期:2018-02-06
中文翻译:

基于上转换纳米胶囊的生物响应和近红外光子共同增强癌症治疗诊断†
开发对肿瘤微环境敏感的纳米治疗学引起了按需癌症诊断和治疗的极大关注。在此,采用简单的锰掺杂策略将介孔二氧化硅涂覆的上转换纳米粒子(UCNP)转化为具有肿瘤响应性生物降解性质的蛋黄样上转换纳米结构。创新纳米载体巨大的内部空间适合阿霉素(DOX)的储存,此外,Mn掺杂的壳对瘤内酸性和还原性敏感,这使得壳能够生物降解,并进一步加速内部Si-O-Si键的断裂。二氧化硅框架。这种肿瘤响应性外壳降解有利于实现肿瘤特异性 DOX 释放。随后,在二氧化硅壳的表面修饰了多金属氧酸盐(POM)纳米团簇,该纳米团簇可以增强光热转换以响应肿瘤的还原性和酸性,从而实现近红外增强的壳降解并防止DOX过早泄漏。POM 产生的热效应与释放的 DOX 的化疗效应相结合,进行协同化学光热疗法。此外,外壳降解使纳米载体尺寸缩小,从而使纳米颗粒更快扩散和更深的肿瘤渗透,这对于改善治疗诊断结果具有重要意义。此外,DOX释放引起的红/绿(R/G)比值急剧下降可用于通过荧光共振能量转移(FRET)方法监测DOX释放含量。Mn释放引起的MRI效应以及Gd 3+ /Yb 3+ /Nd 3+ /Er 3+共掺杂UCNPs在808 nm激光激发下产生的MRI/CT/UCL成像赋予纳米系统多种成像能力,从而实现影像引导的癌症治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号