当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Morning glory resin glycosides as α-glucosidase inhibitors: In vitro and in silico analysis
Phytochemistry ( IF 3.2 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.phytochem.2018.01.012 Daniel Rosas-Ramírez , Sonia Escandón-Rivera , Rogelio Pereda-Miranda
Phytochemistry ( IF 3.2 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.phytochem.2018.01.012 Daniel Rosas-Ramírez , Sonia Escandón-Rivera , Rogelio Pereda-Miranda
|
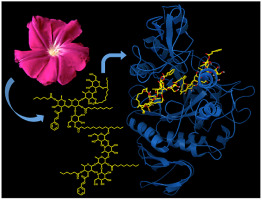
Twenty-seven individual resin glycosides from the morning glory family (Convolvulaceae) were evaluated for their α-glucosidase inhibitory potential. Four of these compounds displayed an inhibitory activity comparable to acarbose, which was used as a positive control. Molecular modeling studies performed by docking analysis were accomplished to predict that the active compounds and acarbose bind to the α-1,4-glucosidase enzyme catalytic site of MAL12 from the yeast Saccharomyces cerevisiae through stable hydrogen bonds primarily with the amino acid residues HIS279 and GLN322. Docking studies with the human maltase-glucoamylase (MGAM) also identified binding modes for resin glycosides inside the catalytic site in the proximity of TYR1251. These results postulate that resin glycosides may be a source of phytotherapeutic agents with antihyperglycemic properties for the prophylaxis and treatment of non-insulin-dependent type 2 diabetes mellitus.
中文翻译:

牵牛花树脂糖苷作为 α-葡萄糖苷酶抑制剂:体外和计算机分析
对来自牵牛花科(旋花科)的 27 种单独的树脂糖苷的 α-葡萄糖苷酶抑制潜力进行了评估。其中四种化合物显示出与用作阳性对照的阿卡波糖相当的抑制活性。通过对接分析进行的分子建模研究旨在预测活性化合物和阿卡波糖通过主要与氨基酸残基 HIS279 和 GLN322 的稳定氢键结合到来自酵母酿酒酵母的 MAL12 的 α-1,4-葡萄糖苷酶催化位点. 与人麦芽糖酶-葡糖淀粉酶 (MGAM) 的对接研究还确定了 TYR1251 附近催化位点内树脂糖苷的结合模式。
更新日期:2018-04-01
中文翻译:

牵牛花树脂糖苷作为 α-葡萄糖苷酶抑制剂:体外和计算机分析
对来自牵牛花科(旋花科)的 27 种单独的树脂糖苷的 α-葡萄糖苷酶抑制潜力进行了评估。其中四种化合物显示出与用作阳性对照的阿卡波糖相当的抑制活性。通过对接分析进行的分子建模研究旨在预测活性化合物和阿卡波糖通过主要与氨基酸残基 HIS279 和 GLN322 的稳定氢键结合到来自酵母酿酒酵母的 MAL12 的 α-1,4-葡萄糖苷酶催化位点. 与人麦芽糖酶-葡糖淀粉酶 (MGAM) 的对接研究还确定了 TYR1251 附近催化位点内树脂糖苷的结合模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号