Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-02-05 , DOI: 10.1016/j.jfluchem.2018.01.014 Amanda M. Pluntze , Eric V. Bukovsky , Matthew R. Lacroix , Brian S. Newell , Christopher D. Rithner , Steven H. Strauss
|
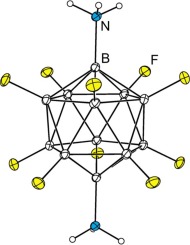
Multiple variations of the reaction conditions reported in 1964 for the diamination of K2B12H12 (potassium dodecahydro-closo-dodecaborate(2−)) with hydroxylamine-O-sulfonic acid were studied to improve the isolated yields of the three isomers of B12H10(NH3)2. Screening the sets of reaction conditions without completely working up each of the reaction mixtures, and therefore without the lengthy separation and isolation of the individual B12H10(NH3)2 isomers for each reaction mixture, was possible by recording 1H-15N HSQC NMR spectra of the crude reaction mixtures. The 1H-dimension of these spectra exhibited narrow, baseline separated resonances for 1,2-, 1,7- and 1,12-B12H10(NH3)2 and for the B12H11(NH3)− intermediate, allowing their relative concentrations to be determined to the nearest percent. The best synthetic conditions resulted in 9, 25, and 6% isolated yields of 1,2-, 1,7- and 1,12-B12H10(NH3)2, respectively. The decafluoro derivatives 1,2-, 1,7-, and 1,12-B12F10(NH3)2 were prepared for the first time by direct fluorination of the B12H10(NH3)2 isomers, either individually or as a mixture subsequently separated by column chromatography similar to the separation and purification of the B12H10(NH3)2 isomers. The structures of 1,7-B12F10(NH2)2·4CH3CONH2, 1,12-B12F10(NH2)2·6CH3CONH2, 1,2-B12H10(NH2)2·1.5CH3CO2CH2CH3, 1,7-B12H10(NH2)2·1.23H2O, 1,12-B12H10(NH2)2·2CH3CN, and solvent-free 1,12-B12H10(NH2)2 were determined by single-crystal X-ray diffraction.
中文翻译:

二氨基硼烷的Deca- B-氟化。1,2-,1,7-和1,12-B 12 H 10(NH 3)2和1,2-,1,7-和1,12-B 12 F 10( NH 3)2
的反应条件的多种变化报道在1964年为K的diamination 2乙12 ħ 12(钾十二氢闭合碳-dodecaborate(2-)),与羟胺ö磺酸进行了研究,以改善的三种异构体的分离产率B 12 H 10(NH 3)2。在不完全处理每种反应混合物的情况下筛选反应条件组,因此无需长时间分离和分离各个B 12 H 10(NH 3)2通过记录粗反应混合物的1 H- 15 N HSQC NMR光谱,可以确定每种反应混合物的异构体。这些光谱的1 H维对1,2-,1,7-和1,12-B 12 H 10(NH 3)2和B 12 H 11(NH 3)-表现出窄的,基线分离的共振-中间体,将其相对浓度确定为最接近的百分比。最佳合成条件导致1,2-,1,7-和1,12-B 12 H 10(NH 3)2的分离产率为9、25和6%, 分别。十氟衍生物1,2-,1,7-和1,12-B 12 F 10(NH 3)2是通过B 12 H 10(NH 3)2异构体的直接氟化而首次制备的。单独地或作为混合物随后通过柱色谱法进行分离,类似于B 12 H 10(NH 3)2异构体的分离和纯化。1,7-B的结构12 ˚F 10(NH 2)2 ·4CH 3 CONH 2,1,12-乙12 ˚F 10(NH 2)2 ·6CH 3 CONH 2,1,2--B 12 ħ 10(NH 2)2 ·1.5CH 3 CO 2 CH 2 CH 3,1,7--B 12 ħ 10(NH 2)2 ·1.23H 2 O,1,12-B 12 H 10(NH 2)2 ·2CH 3 CN和无溶剂1,12-B 12 H 10(NH 2)2 通过单晶X射线衍射确定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号