当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fabrication of mesoporous La3Ga5GeO14:Cr3+,Zn2+ persistent luminescence nanocarriers with super-long afterglow for bioimaging-guided in vivo drug delivery to the gut†
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1039/c7tb02759a Dong-Dong Zhang 1, 2, 3, 4, 5 , Jing-Min Liu 5, 6, 7, 8, 9 , Nan Song 1, 2, 3, 4, 5 , Yao-Yao Liu 1, 2, 3, 4, 5 , Meng Dang 1, 2, 3, 4, 5 , Guo-Zhen Fang 1, 2, 3, 4, 5 , Shuo Wang 5, 6, 7, 8, 9
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1039/c7tb02759a Dong-Dong Zhang 1, 2, 3, 4, 5 , Jing-Min Liu 5, 6, 7, 8, 9 , Nan Song 1, 2, 3, 4, 5 , Yao-Yao Liu 1, 2, 3, 4, 5 , Meng Dang 1, 2, 3, 4, 5 , Guo-Zhen Fang 1, 2, 3, 4, 5 , Shuo Wang 5, 6, 7, 8, 9
Affiliation
|
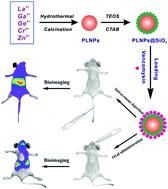
Infection by pathogens has always been a major threat to human health, and various drugs have been explored and designed to kill pathogens in the past decades. However, pathogens are evolving faster than the development of new antibiotics, and increasing doses are resulting in increasing side effects and toxicity; this has prompted us to exert effort toward the development of advanced drug carriers for precise delivery of antibiotics. In this study, with the involvement of persistent luminescence nanophosphors (PLNPs) as the emission core, we propose an antibiotic nanocarrier for in vivo delivery of vancomycin to intestinal bacteria via bioimaging guidance. The synthesized PLNPs were coated with mesoporous silica for vancomycin adsorption (NPs@SiO2@Van) and used as an efficient nanocarrier for direct vancomycin delivery and in vivo imaging with low cytotoxicity toward MC38 cell lines. Additionally, we detected the luminescence signals of NPs@SiO2@Van during their use as nanocarriers for vancomycin and accurately obtained the biodistribution of NPs@SiO2@Van in situ and in real time with neglectable auto-fluorescence from the animal body. For the first time, bioimaging-guided in vivo drug delivery to gut bacteria was realized in the present work. The outstanding luminescence features and excellent biocompatibility and structural stability of PLNPs may open new horizons in the development of nanocarriers for nano-diagnosis/therapy and in vivo studies of intestinal microorganisms.
中文翻译:

具有超长余辉 的介孔La 3 Ga 5 GeO 14:Cr 3+,Zn 2+持久发光纳米载体的制备,用于生物成像引导的体内药物向肠道的递送†
病原体的感染一直是对人类健康的主要威胁,在过去的几十年中,已经开发出各种药物来杀死病原体。但是,病原体的发展比新抗生素的发展快,剂量增加导致副作用和毒性增加。这促使我们致力于开发用于精确递送抗生素的先进药物载体。在这项研究中,以持久发光纳米磷光体(PLNPs)为发射核心,我们提出了一种抗生素纳米载体,用于通过生物成像指导将万古霉素体内递送至肠道细菌。合成的PLNPs用中孔二氧化硅包被以吸收万古霉素(NPs @ SiO 2@Van),并用作直接万古霉素递送和体内成像的有效纳米载体,对MC38细胞系的细胞毒性低。此外,我们检测到的NP的发光信号@的SiO 2 @Van它们作为纳米载体为万古霉素在使用过程中,准确地得到的NP @的SiO的生物分布2 @Van原位并与从动物体中不容忽视的自发荧光实时。首次在体内进行生物成像引导在目前的工作中已经实现了向肠道细菌的药物递送。PLNPs的出色发光特性,出色的生物相容性和结构稳定性,可为用于纳米诊断/治疗和肠道微生物体内研究的纳米载体的开发开辟新的视野。
更新日期:2018-02-05
中文翻译:

具有超长余辉 的介孔La 3 Ga 5 GeO 14:Cr 3+,Zn 2+持久发光纳米载体的制备,用于生物成像引导的体内药物向肠道的递送†
病原体的感染一直是对人类健康的主要威胁,在过去的几十年中,已经开发出各种药物来杀死病原体。但是,病原体的发展比新抗生素的发展快,剂量增加导致副作用和毒性增加。这促使我们致力于开发用于精确递送抗生素的先进药物载体。在这项研究中,以持久发光纳米磷光体(PLNPs)为发射核心,我们提出了一种抗生素纳米载体,用于通过生物成像指导将万古霉素体内递送至肠道细菌。合成的PLNPs用中孔二氧化硅包被以吸收万古霉素(NPs @ SiO 2@Van),并用作直接万古霉素递送和体内成像的有效纳米载体,对MC38细胞系的细胞毒性低。此外,我们检测到的NP的发光信号@的SiO 2 @Van它们作为纳米载体为万古霉素在使用过程中,准确地得到的NP @的SiO的生物分布2 @Van原位并与从动物体中不容忽视的自发荧光实时。首次在体内进行生物成像引导在目前的工作中已经实现了向肠道细菌的药物递送。PLNPs的出色发光特性,出色的生物相容性和结构稳定性,可为用于纳米诊断/治疗和肠道微生物体内研究的纳米载体的开发开辟新的视野。











































 京公网安备 11010802027423号
京公网安备 11010802027423号