当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dioxygen Activation by Nonheme Diiron Enzymes: Diverse Dioxygen Adducts, High-Valent Intermediates, and Related Model Complexes
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.chemrev.7b00457 Andrew J Jasniewski 1 , Lawrence Que 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.chemrev.7b00457 Andrew J Jasniewski 1 , Lawrence Que 1
Affiliation
|
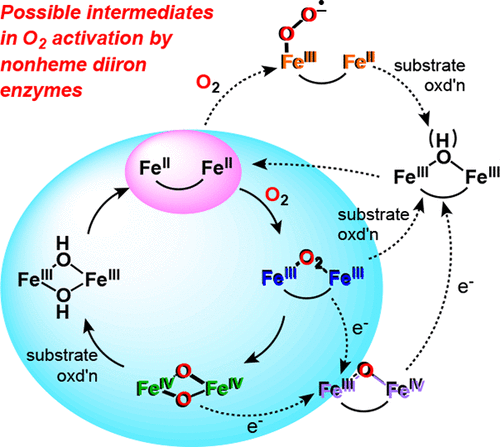
A growing subset of metalloenzymes activates dioxygen with nonheme diiron active sites to effect substrate oxidations that range from the hydroxylation of methane and the desaturation of fatty acids to the deformylation of fatty aldehydes to produce alkanes and the six-electron oxidation of aminoarenes to nitroarenes in the biosynthesis of antibiotics. A common feature of their reaction mechanisms is the formation of O2 adducts that evolve into more reactive derivatives such as diiron(II,III)-superoxo, diiron(III)-peroxo, diiron(III,IV)-oxo, and diiron(IV)-oxo species, which carry out particular substrate oxidation tasks. In this review, we survey the various enzymes belonging to this unique subset and the mechanisms by which substrate oxidation is carried out. We examine the nature of the reactive intermediates, as revealed by X-ray crystallography and the application of various spectroscopic methods and their associated reactivity. We also discuss the structural and electronic properties of the model complexes that have been found to mimic salient aspects of these enzyme active sites. Much has been learned in the past 25 years, but key questions remain to be answered.
中文翻译:

非血红素二铁酶的分子氧活化:多种分子氧加合物、高价中间体和相关模型复合物
越来越多的金属酶子集通过非血红素二铁活性位点激活双氧,以影响底物氧化,范围从甲烷的羟基化和脂肪酸的去饱和到脂肪醛的去甲酰化以产生烷烃以及氨基芳烃到硝基芳烃的六电子氧化抗生素的生物合成。它们反应机制的一个共同特征是形成O 2加合物,并演变成更具反应性的衍生物,例如二铁(II,III)-超氧、二铁(III)-过氧、二铁(III,IV)-氧和二铁(III,IV)-氧。 IV)-氧代物种,执行特定的底物氧化任务。在这篇综述中,我们调查了属于这个独特子集的各种酶以及底物氧化的机制。我们研究了 X 射线晶体学和各种光谱方法的应用及其相关反应性所揭示的反应中间体的性质。我们还讨论了模型复合物的结构和电子特性,这些模型复合物已被发现模仿这些酶活性位点的显着方面。过去 25 年我们学到了很多东西,但关键问题仍有待解答。
更新日期:2018-02-05
中文翻译:

非血红素二铁酶的分子氧活化:多种分子氧加合物、高价中间体和相关模型复合物
越来越多的金属酶子集通过非血红素二铁活性位点激活双氧,以影响底物氧化,范围从甲烷的羟基化和脂肪酸的去饱和到脂肪醛的去甲酰化以产生烷烃以及氨基芳烃到硝基芳烃的六电子氧化抗生素的生物合成。它们反应机制的一个共同特征是形成O 2加合物,并演变成更具反应性的衍生物,例如二铁(II,III)-超氧、二铁(III)-过氧、二铁(III,IV)-氧和二铁(III,IV)-氧。 IV)-氧代物种,执行特定的底物氧化任务。在这篇综述中,我们调查了属于这个独特子集的各种酶以及底物氧化的机制。我们研究了 X 射线晶体学和各种光谱方法的应用及其相关反应性所揭示的反应中间体的性质。我们还讨论了模型复合物的结构和电子特性,这些模型复合物已被发现模仿这些酶活性位点的显着方面。过去 25 年我们学到了很多东西,但关键问题仍有待解答。











































 京公网安备 11010802027423号
京公网安备 11010802027423号