Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-02-05 , DOI: 10.1016/j.jconrel.2018.02.007 Abraham R. Tzafriri , Fernando Garcia-Polite , Xiaojian Li , John Keating , Josep-Maria Balaguer , Brett Zani , Lynn Bailey , Peter Markham , Timothy C. Kiorpes , Wenda Carlyle , Elazer R. Edelman
|
Background
Innovations in drug eluting stent designs make it increasingly important to develop models for differentiating performance through spatial definition of drug, receptor binding and cell state.
Methods
Two designs of sirolimus analog eluting stents were implanted into porcine coronary arteries for 28, 60 or 90 days (n = 9/time point), durable coating (Xience) and deployable absorbable coating (MiStent). Explanted arteries were evaluated for drug content (n = 3/time point) by LC-MS/MS and for drug and target protein (mTOR) distributions by immunofluorescence (IF, n = 6/time point). A computational model was developed to predict drug release and arterial distribution maps.
Results
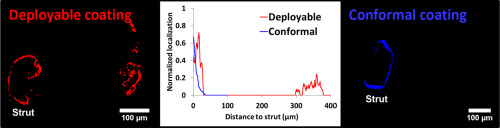
Both stents released the majority of drug load by 28 days, with different tissue retention efficiencies (91.4 ± 4.9% MiStent versus 21.5 ± 1.9% Xience, P < 0.001). Computational modeling of MiStent coating deployment and microcrystal dissolution recapitulated in vivo drug release and net tissue content and predicted that >98.5% of deployed drug remains crystalline through 90 days. Immunofluorescence and computational modeling showed peristrut drug localization for both stents, with similar peaks, but high interstrut levels only at sites of coating deployment from the absorbable coating. Co-localization of mTOR-IF with drug-IF for both devices showed persistent drug effects, though with differential drug–receptor pharmacokinetics.
Conclusions
Immunofluorescence and computational modeling provide insights into drug distribution and binding status that can help differentiate drug delivery technologies. Herein we found that tissue deployment of slow dissolving crystalline drug particles results in temporally and spatially more uniform drug delivery to interstrut zones that might otherwise be under-dosed without excess peristrut drug.
中文翻译:

定义基于支架的药物释放后的药物和靶蛋白分布:耐用涂层与可展开涂层
背景
药物洗脱支架设计的创新使得通过药物的空间定义,受体结合和细胞状态来区分性能的模型变得越来越重要。
方法
将两种设计的西罗莫司类似物洗脱支架植入猪冠状动脉中28、60或90天(n = 9 /时间点),耐用涂层(Xience)和可展开的可吸收涂层(MiStent)。通过LC-MS / MS评估移植动脉的药物含量(n = 3 /时间点),并通过免疫荧光法评估药物和靶蛋白(mTOR)的分布(IF,n = 6 /时间点)。开发了一种计算模型来预测药物释放和动脉分布图。
结果
两种支架在28天时释放了大部分药物负荷,具有不同的组织保留效率(MiStent为91.4±4.9%,Xience为21.5±1.9%,P <0.001)。MiStent涂层部署和微晶溶解的计算模型概括了体内药物释放和净组织含量,并预测超过98.5%的已部署药物在90天内保持结晶状态。免疫荧光和计算模型显示两个支架的支架周围药物定位,具有相似的峰,但仅在可吸收涂层从涂层展开的部位具有高支架水平。尽管具有不同的药物-受体药代动力学,两种设备的mTOR-IF与drug-IF的共定位显示出持续的药物作用。
结论
免疫荧光和计算模型可洞悉药物分布和结合状态,从而有助于区分药物输送技术。在本文中,我们发现缓慢溶解的结晶药物颗粒的组织展开导致在时间和空间上更均匀地将药物递送至间质区域,否则这些间质区域的剂量可能不足而没有过多的间质药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号