当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oral drug suitability parameters†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1039/c7md00586e M C Wenlock 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1039/c7md00586e M C Wenlock 1
Affiliation
|
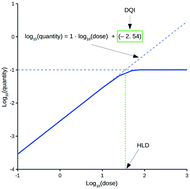
Assessing the oral drug suitability of compounds as early as possible within drug discovery is an important objective. This study describes a methodology that attempts to simplify the evaluation of compounds based on their in vivo quantity levels within a mammalian body, represented using a mathematical model that imposes a time limitation on oral absorption and assumes non-instantaneous drug distribution between plasma and tissue. This simplification results in two new oral drug suitability parameters that can quantitatively relate oral dose to in vivo exposure for compounds with vastly different tendencies in terms of absorption into, and elimination from, the body. Consequently, the complexities associated with evaluating a compound's oral drug suitability are simplified to an assessment of these two new parameters. Application of this methodology at the virtual design stage is discussed, along with functionality that accounts for uncertainty related to a compound's distribution kinetics and errors associated to in silico QSAR predictions for the required input data.
中文翻译:

口服药物适宜性参数†
在药物发现过程中尽早评估化合物的口服药物适用性是一个重要目标。这项研究描述了一种方法,试图根据化合物在哺乳动物体内的体内数量水平来简化评估,该方法使用数学模型来表示,该数学模型对口服吸收施加时间限制,并假设血浆和组织之间的非瞬时药物分布。这种简化产生了两个新的口服药物适宜性参数,可以定量地将口服剂量与体内吸收和消除倾向截然不同的化合物的体内暴露联系起来。因此,与评估化合物的口服药物适用性相关的复杂性被简化为对这两个新参数的评估。讨论了该方法在虚拟设计阶段的应用,以及解释与化合物分布动力学相关的不确定性以及与所需输入数据的计算机QSAR 预测相关的误差的功能。
更新日期:2018-02-05
中文翻译:

口服药物适宜性参数†
在药物发现过程中尽早评估化合物的口服药物适用性是一个重要目标。这项研究描述了一种方法,试图根据化合物在哺乳动物体内的体内数量水平来简化评估,该方法使用数学模型来表示,该数学模型对口服吸收施加时间限制,并假设血浆和组织之间的非瞬时药物分布。这种简化产生了两个新的口服药物适宜性参数,可以定量地将口服剂量与体内吸收和消除倾向截然不同的化合物的体内暴露联系起来。因此,与评估化合物的口服药物适用性相关的复杂性被简化为对这两个新参数的评估。讨论了该方法在虚拟设计阶段的应用,以及解释与化合物分布动力学相关的不确定性以及与所需输入数据的计算机QSAR 预测相关的误差的功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号