Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-04 , DOI: 10.1016/j.mcat.2017.12.039 Cecilia C. Torres , Verónica A. Jiménez , Cristian H. Campos , Joel B. Alderete , Robinson Dinamarca , Tatiana M. Bustamente , Barbara Pawelec
|
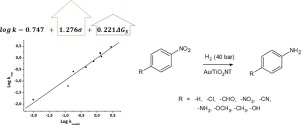
Gold nanoparticles supported on titania nanotubes (TiO2NT) were synthesized and employed as an efficient catalysts for the selective hydrogenation of nitrobenzenes. Materials characterization by N2 adsorption-desorption isotherms, XRD, HRTEM, DRS UV–vis, and XPS revealed that structured materials with high metal dispersion were obtained. The catalytic properties of these materials were tested using nitrobenzene and eight p-substituted analogs as model compounds with the aim of gaining insight into the role of electron-withdrawing and electron-donating substituents on the rate and selectivity of hydrogenation to the corresponding p-substituted anilines. Catalytic data showed pseudo first order kinetics for all compounds, with minimum formation of reaction intermediates and absence of condensation side products. Quantum chemical computational calculations demonstrated that the experimental kinetic constants (k) for the series of nitrobenzenes under study were described by a multilinear regression equation using the substituent Hammet sigma constant (σ), and the calculated solvation energy of the reactants (ΔGsolv) as predictor variables. The catalyst activity-structure correlation revealed that electronic effects are critical for the reaction kinetics, and that electron-withdrawing groups increase the hydrogenation rates over electron-donating substituents. Solvation plays also a relevant role as less solvated species interact better with the catalyst surface and react faster than highly solvated substrates. These results are valuable to design novel efficient strategies for the selective hydrogenation of nitrocompounds.
中文翻译:

负载在TiO 2纳米管上的金催化剂,用于对位取代的硝基苯的选择性加氢
合成了负载在二氧化钛纳米管(TiO 2 NT)上的金纳米颗粒,并将其用作硝基苯选择性加氢的有效催化剂。通过N 2吸附-解吸等温线,XRD,HRTEM,DRS UV-vis和XPS对材料进行表征,表明获得了具有高金属分散性的结构化材料。使用硝基苯这些材料的催化性能进行了测试和八个p取代的类似物为模型化合物与洞悉的作用的目的的吸电子和给电子取代基上的速率和氢化的选择性为相应的p取代的苯胺。催化数据显示了所有化合物的拟一级动力学,反应中间体的形成最少,并且没有缩合副产物。量子化学计算结果表明,所研究的一系列硝基苯的实验动力学常数(k)由使用取代基Hammet sigma常数(σ)的多元线性回归方程和计算出的反应物的溶剂化能(ΔGsolv)来描述。)作为预测变量。催化剂活性-结构的相关性表明,电子效应对于反应动力学至关重要,并且吸电子基团比给电子取代基提高了氢化速率。溶剂化也起着重要的作用,因为与高度溶剂化的底物相比,较少溶剂化的物质与催化剂表面的相互作用更好,反应速度也更快。这些结果对于设计用于硝基化合物选择性加氢的新颖有效策略是有价值的。










































 京公网安备 11010802027423号
京公网安备 11010802027423号