Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-04 , DOI: 10.1016/j.mcat.2018.01.003 Ellie L. Uzunova
|
The decоmposition of co-adsorbed nitric oxide (NO) and nitrogen dioxide (NO2) on copper-exchanged SAPO−34 and SSZ−13 proceeds with lower activation barriers in all intermediate steps, as compared to the DeNOx reaction of dinitrosyls. Upon co-adsorption on copper cations, NO and NO2 form an η1O adsorption complex with N
N bond and in the presence of Brønsted acid sites, oxygen atoms from the adsorption complex bind to the framework via hydrogen bonds. Direct protonation of the adsorption complex may occur at either the NO2 end (ONNO2H), or at the NO-end, (HONNO2). Upon ONNO2H formation, the N
N bond is weakened, while NO protonation (HONNO2) strengthens the N
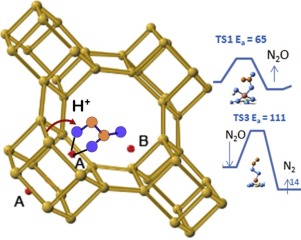
N bond and a nearly cyclic structure with the transition metal cation is formed. Two of the reaction steps have high energy barriers: the release of N2O on SSZ−13 after protonation transfer to ONNO2, and the decomposition of N2O on a ZeolCuO site. The first reaction step is the release of N2O and protonation of the adsorption complex plays a detrimental role. The activation barrier obtained by intrinsic reaction coordinate calculations is higher for SSZ−13 (116 kJ mol−1), but much smaller for SAPO−34 (65 kJ mol−1). After desorption of molecular oxygen, the N2O molecule is re-adsorbed and its dissociation with release of molecular nitrogen is assisted by hydrogen bond formation, which stabilizes the transition states and lowers the energy barriers. After the release of molecular nitrogen, surface copper oxide, ZeolCuO site is left and it is more reactive than copper hydroxyl, ZeolCu(OH)+.
中文翻译:

布朗斯台德酸位存在下二氧化氮与一氧化氮共吸附和DeNO x在Cu-SAPO-34和Cu-SSZ-13上的理论研究
与二亚硝酰基的DeNOx反应相比,铜交换的SAPO-34和SSZ-13上共吸附一氧化氮(NO)和二氧化氮(NO 2)的沉积在所有中间步骤中均具有较低的活化势垒。在上铜阳离子,NO和NO共吸附2形式的η 1 ö吸附复合用N
N键和在通过氢键的布朗斯台德酸位点,氧原子存在下从吸附复杂结合的框架。吸附复合物的直接质子化可发生在NO 2端(ONNO 2 H)或NO端(HONNO 2)。形成ONNO 2 H后,N
N键减弱,而NO质子化(HONNO 2)增强N
N键,并与过渡金属阳离子形成近乎环状的结构。其中两个反应步骤具有较高的能垒:质子转移到ONNO 2后,SSZ-13上N 2 O的释放以及ZeolCuO位点上N 2 O的分解。第一步反应是释放N 2 O,吸附复合物的质子化起有害作用。通过本征反应坐标计算获得的活化势垒对于SSZ-13(116 kJ mol -1)较高,但对于SAPO-34(65 kJ mol -1)小得多。分子氧解吸后,N 2O分子被重新吸附,并通过氢键的形成辅助其与分子氮的释放解离,从而稳定了过渡态并降低了能垒。释放出分子氮后,表面的氧化铜,ZeolCuO位点得以保留,并且比羟基铜的ZeolCu(OH)+更具反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号