Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-04 , DOI: 10.1016/j.mcat.2018.01.002 Mohamad Hekarl Uzir , Nazalan Najimudin
|
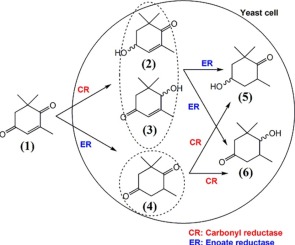
Baker’s yeast has been well-known to have the ability to reduce a variety of substrates into many optically active compounds. One of the important chemicals is (4R,6R)-4-hydroxy-2,2,6-trimethylcyclohexanone or in short, (4R,6R)-actinol, a product formed from the reduction of 2,6,6-trimethylcyclohex-2-ene-1,4-dione, (ketoisophorone). The work has successfully characterized the route of the reaction during the conversion mediated by Saccharomyces cerevisiae and it was also experimentally observed the importance of cofactor (NADH/NADPH) availability and stability during the biotransformation. The presence of cofactor has been proven to assist the product formation by keeping a continuous flow of hydrogen ions to and from the reduction system, while the amount of enzymes present determined the rate of both intermediates and product formed during the course of biotransformation. Results of the growing cells, particularly during the exponential phase of the growth significantly differ to that of the stationary phase system in terms of the formation of the intermediates and the final product. For the growing cell biotransformation, the route only stopped at the intermediate compound (4) during the course of the 30 h reaction, whereas for the stationary phase biotransformation, compound (5) was readily formed in a range of time between 2 and 12 h of reaction, depending on the amount of cells available and glucose supplied to the system. Within the yeast cell, there are two responsible enzymes that mediate the biotransformation; carbonyl reductase and enoate reductase that work both in parallel and sequential to produce a chiral alcohol of (4R,6R)-actinol.
中文翻译:

关于酿酒酵母介导的2,6,6-三甲基环己-2-烯-1,4-二酮的立体选择性生物转化的双酶行为
众所周知,贝克酵母具有将多种底物还原为许多光学活性化合物的能力。重要的化学物质之一是(4 R,6 R)-4-羟基-2,2,6-三甲基环己酮,或者简称为(4 R,6 R)-肌醇,它是由2,6还原而成的, 6-三甲基环己-2-烯-1,4-二酮(酮异佛尔酮)这项工作成功地表征了由酿酒酵母介导的转化过程中的反应途径并且还通过实验观察了生物转化过程中辅因子(NADH / NADPH)的有效性和稳定性的重要性。辅因子的存在已被证明通过保持氢离子往返于还原系统的连续流动而有助于产物形成,而存在的酶的量决定了生物转化过程中中间体和产物形成的速率。就中间产物和最终产物的形成而言,生长细胞的结果,特别是在生长的指数期期间,与固定相系统的结果显着不同。对于不断增长的细胞生物转化,该途径仅在中间化合物处停止(4)在30小时的反应过程中,而对于固定相生物转化,根据可用的细胞数量和向系统提供的葡萄糖,在反应2至12小时的时间内很容易形成化合物(5) 。在酵母细胞内,有两种负责任的酶介导生物转化。羰基还原酶和烯酸酯还原酶并行和顺序工作,生成(4 R,6 R)-肌动醇的手性醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号