当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydride-Triel Bonds
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2018-02-05 , DOI: 10.1002/jcc.25178 Mirosław Jabłoński 1
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2018-02-05 , DOI: 10.1002/jcc.25178 Mirosław Jabłoński 1
Affiliation
|
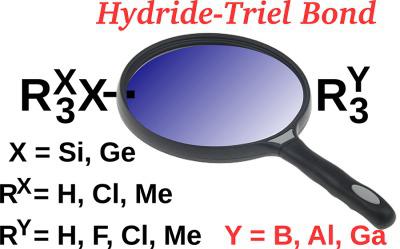
In this article, we present the results of our comprehensive studies of 72 dimers of the R3XXH⋯YR3Y type (X = Si, Ge; Y = B, Al, Ga; RX = H, Cl, Me; RY = H, F, Cl, Me) and featuring hydride‐triel bonds (i.e., charge‐inverted hydrogen bonds). Influence of X and Y atoms as well as RX and RY substituents on various properties of these dimers is investigated in detail. In particular the strength of the H⋯Y hydride‐triel bonds is paid a close attention and it is shown that hydride‐triel bonds can be strong enough to considerably determine structure and properties of molecular systems. In addition, properties of the investigated dimers are largely governed by the charge transfer from the Lewis base to the Lewis acid, which is particularly important if more bulky and polarizable RY and Y atoms are present in the YR3Y molecule. Several excellent linear (R2 close to 1) and exponential correlations between pairs of diverse parameters are presented. Few instances are discussed where somewhat unexpected bond paths exist between two atoms featuring partial negative charges (e.g., between hydride hydrogen and halogen and between lateral sides of two halogens) showing that in some cases a bond path prefers to link two closely spaced electron‐rich atoms instead of two atoms that are expected to form a bond. © 2018 Wiley Periodicals, Inc.
中文翻译:

氢化物-Triel 键
在本文中,我们展示了对 R3XXH⋯YR3Y 型(X = Si、Ge;Y = B、Al、Ga;RX = H、Cl、Me;RY = H、F、 Cl, Me) 并具有氢化物-三烯键(即电荷反转氢键)。详细研究了 X 和 Y 原子以及 RX 和 RY 取代基对这些二聚体的各种性质的影响。特别是 H⋯Y 氢化物 - 三烯键的强度受到密切关注,并且表明氢化物 - 三烯键的强度足以显着确定分子系统的结构和性质。此外,所研究的二聚体的性质在很大程度上取决于从路易斯碱到路易斯酸的电荷转移,如果 YR3Y 分子中存在更大且可极化的 RY 和 Y 原子,这一点尤其重要。介绍了不同参数对之间的几个极好的线性(R2 接近 1)和指数相关性。很少有实例讨论了在具有部分负电荷的两个原子之间(例如,氢化物氢和卤素之间以及两个卤素的侧面之间)存在一些意想不到的键路径的情况,表明在某些情况下,键路径更喜欢连接两个相距很近的富电子原子而不是预期形成键的两个原子。© 2018 Wiley Periodicals, Inc. 氢和卤素之间以及两个卤素的侧面之间)表明在某些情况下,键路径更喜欢连接两个紧密间隔的富电子原子,而不是预期形成键的两个原子。© 2018 Wiley Periodicals, Inc. 氢和卤素之间以及两个卤素的侧面之间)表明在某些情况下,键路径更喜欢连接两个紧密间隔的富电子原子,而不是预期形成键的两个原子。© 2018 Wiley Periodicals, Inc.
更新日期:2018-02-05
中文翻译:

氢化物-Triel 键
在本文中,我们展示了对 R3XXH⋯YR3Y 型(X = Si、Ge;Y = B、Al、Ga;RX = H、Cl、Me;RY = H、F、 Cl, Me) 并具有氢化物-三烯键(即电荷反转氢键)。详细研究了 X 和 Y 原子以及 RX 和 RY 取代基对这些二聚体的各种性质的影响。特别是 H⋯Y 氢化物 - 三烯键的强度受到密切关注,并且表明氢化物 - 三烯键的强度足以显着确定分子系统的结构和性质。此外,所研究的二聚体的性质在很大程度上取决于从路易斯碱到路易斯酸的电荷转移,如果 YR3Y 分子中存在更大且可极化的 RY 和 Y 原子,这一点尤其重要。介绍了不同参数对之间的几个极好的线性(R2 接近 1)和指数相关性。很少有实例讨论了在具有部分负电荷的两个原子之间(例如,氢化物氢和卤素之间以及两个卤素的侧面之间)存在一些意想不到的键路径的情况,表明在某些情况下,键路径更喜欢连接两个相距很近的富电子原子而不是预期形成键的两个原子。© 2018 Wiley Periodicals, Inc. 氢和卤素之间以及两个卤素的侧面之间)表明在某些情况下,键路径更喜欢连接两个紧密间隔的富电子原子,而不是预期形成键的两个原子。© 2018 Wiley Periodicals, Inc. 氢和卤素之间以及两个卤素的侧面之间)表明在某些情况下,键路径更喜欢连接两个紧密间隔的富电子原子,而不是预期形成键的两个原子。© 2018 Wiley Periodicals, Inc.



























 京公网安备 11010802027423号
京公网安备 11010802027423号