Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-02-03 , DOI: 10.1016/j.jcat.2017.12.026 Yang Song , Udishnu Sanyal , Dhananjai Pangotra , Jamie D. Holladay , Donald M. Camaioni , Oliver Y. Gutiérrez , Johannes A. Lercher
|
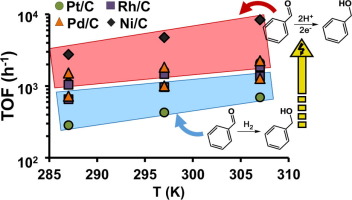
Selective reduction of benzaldehyde to benzyl alcohol (model reaction for low-temperature stabilization of bio-oil) on C-supported Pt, Rh, Pd, and Ni in aqueous phase was conducted using either H2 (thermal catalytic hydrogenation, TCH) or hydrogen generated in situ electrocatalytically (electrocatalytic hydrogenation, ECH). In TCH, the intrinsic activity of the metals at room temperature and 1 bar H2 increased in the sequence Pt/C < Rh/C ≤ Pd/C, while Ni/C is inactive. At these conditions, the coverage of benzaldehyde is high while the coverage of adsorbed H is low and the reaction follows a Langmuir-Hinshelwood mechanism. All tested metals were active in ECH of benzaldehyde above the onset potentials of the H2 evolution reaction (HER). Thus, hydrogenation competes with HER. The relative rates of H reacting to H2 and H addition to benzaldehyde determines the selectivity to ECH and HER. Accordingly, the selectivity of the metals towards ECH increases in the order as follows: Ni/C < Pt/C < Rh/C < Pd/C. The latter having ECH selectivity around 99%. In ECH, the intrinsic activities of all tested metals were higher and the activation energies of benzaldehyde hydrogenation were lower than in TCH.
中文翻译:

碳载金属通过电催化和热催化加氢苯甲醛
使用H 2(热催化加氢,TCH)或氢气将苯甲醛选择性还原为C负载的Pt,Rh,Pd和Ni上的苯甲醇(生物油的低温稳定模型反应)。电催化原位生成(电催化加氢,ECH)。在TCH中,金属在室温和1 bar H 2下的固有活性按Pt / C <Rh / C≤Pd / C的顺序增加,而Ni / C则无活性。在这些条件下,苯甲醛的覆盖率较高,而吸附的H的覆盖率较低,反应遵循Langmuir-Hinshelwood机理。在H 2的起始电位以上,所有测试的金属在苯甲醛的ECH中均具有活性。进化反应(HER)。因此,氢化与HER竞争。H与H 2反应和H加至苯甲醛的相对速率决定了对ECH和HER的选择性。因此,金属对ECH的选择性按以下顺序增加:Ni / C <Pt / C <Rh / C <Pd / C。后者的ECH选择性约为99%。在ECH中,与TCH中相比,所有测试金属的固有活性都较高,苯甲醛加氢的活化能较低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号