Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-02-03 , DOI: 10.1016/j.jcat.2017.12.035 Alina Moscu , Christina Theodoridi , Luis Cardenas , Chloé Thieuleux , Debora Motta-Meira , Giovanni Agostini , Yves Schuurman , Frederic Meunier
|
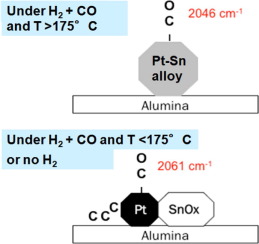
Identifying the causes of the segregation of Pt-based alloy nanoparticles is crucial for the design and operation of the corresponding catalysts and electrodes. This article describes a yet unreported mode of alloy segregation upon exposure to CO. In situ IR studies indicated that Pt-Sn alloy nanoparticles were not stable under (i) H2-free CO at any temperature or (ii) CO/H2 mixtures at temperatures below ca. 450 K. This was rationalized by the ability of Pt to dissociate CO. The oxygen adatoms readily reacted with metallic Sn to form a SnOx species, leading to Pt-Sn segregation, alongside carbon deposition. XPS and XANES analyses confirmed Sn reoxidation. While a contamination by traces of O2 at the sub-ppm level cannot be excluded, the data reported indicate that the structural modifications undergone by the Pt-Sn nanoparticles are more consistent with a reaction involving CO rather than one involving O2. In particular, the XPS analysis after CO exposure revealed an increased fraction of graphitic carbon, while that of oxidized carbon decreased. Thermodynamic calculations indicate that the oxidation of tin by CO, with concomitant carbon formation (Sn + CO → SnO + C) is as favourable as Boudouard reaction (2 CO → CO2 + C) at low temperatures. Alloy stability in the presence of CO must therefore be a concern when CO dissociation is possible and one of the alloyed metal is oxophilic.
中文翻译:

Pt-Sn纳米颗粒上的CO解离引发Sn氧化和合金偏析
确定Pt基合金纳米颗粒偏析的原因对于相应催化剂和电极的设计和操作至关重要。本文介绍了一种尚未报道的暴露于CO时合金偏析的模式。原位红外研究表明,Pt-Sn合金纳米颗粒在(i)在任何温度下都不含H 2的CO或(ii)CO / H 2混合物下均不稳定。温度低于约 450K。这是由于Pt分解CO的能力而合理的。氧原子容易与金属Sn反应形成SnOx物种,导致Pt-Sn偏析以及碳沉积。XPS和XANES分析证实了锡的再氧化。虽然被痕量的O 2污染在亚ppm水平不能排除,报道的数据表明,Pt-Sn纳米颗粒经历的结构修饰与涉及CO而不是涉及O 2的反应更一致。特别是,CO暴露后的XPS分析表明,石墨碳的含量增加,而氧化碳的含量下降。热力学计算表明, 在低温下,CO氧化锡并伴有碳形成(Sn + CO→SnO + C)与Boudouard反应(2 CO→CO 2 + C)一样有利。因此,当可能发生CO解离并且其中一种合金金属具有亲氧性时,必须考虑存在CO时的合金稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号