Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-02-03 , DOI: 10.1016/j.jcat.2018.01.004 Sai Zhang , Zhaoming Xia , Ting Ni , Zhiyun Zhang , Yuanyuan Ma , Yongquan Qu
|
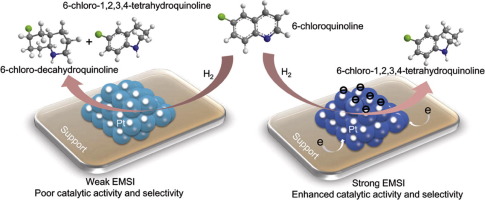
Selectively catalytic hydrogenation of various quinolines in the presence of other reducible groups into the 1,2,3,4-tetrahydroquinoline compounds under mild conditions is a particular challenge for the heterogeneous catalysts due to the natural chemical stability of quinolines and poor chemoselectivity. Pt-based catalysts generally deliver good catalytic activity and selectivity for hydrogenation of quinolines with other reducible groups. However, the over-hydrogenated by-products are also often observed. Herein, Pt nanoparticles supported on CeO2 nanorods (Pt/NR-CeO2) realized highly efficient and chemoselective hydrogenation of various functionalized quinolines at room temperature. The unusual catalytic performance can be attributed to the strong electronic metal-support interactions (EMSI) between Pt and NR-CeO2 with the abundant surface oxygen vacancy, resulting in the enhanced electronic density of Pt nanoparticles. Subsequently, the modulated electronic structure of Pt enables the efficient hydrogen activation at room temperature, leading to a TOF of 546 h−1 of Pt/NR-CeO2 for the selective hydrogenation of 6-chloroquinolines. Density functional theory calculations also reveal that the high electron density of Pt benefits the desorption of the hydrogenated products and thus avoids the over-hydrogenation effectively.
中文翻译:

Pt / CeO 2的强电子金属-载体相互作用可在室温下对喹啉进行有效和选择性的氢化
由于喹啉的天然化学稳定性和差的化学选择性,在温和条件下,在存在其他可还原基团的情况下,将各种喹啉选择性催化氢化为1,2,3,4-四氢喹啉化合物是非均相催化剂的一个特殊挑战。基于Pt的催化剂通常为喹啉与其他可还原基团的氢化提供良好的催化活性和选择性。但是,也经常观察到过度氢化的副产物。本文中,负载在CeO 2纳米棒上的Pt纳米颗粒(Pt / NR -CeO 2)在室温下实现了各种功能化喹啉的高效和化学选择性加氢。异常的催化性能可以归因于Pt和NR -CeO 2之间的强电子金属-载体相互作用(EMSI),以及大量的表面氧空位,从而导致Pt纳米粒子的电子密度提高。随后,Pt的调制电子结构实现了在室温下有效的氢活化,从而导致Pt / NR -CeO 2的TOF为546 h -1用于6-氯喹啉的选择性加氢。密度泛函理论计算还表明,Pt的高电子密度有利于氢化产物的解吸,从而有效避免了过度氢化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号