Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-02-03 , DOI: 10.1016/j.jconrel.2018.02.001 Qingpo Li , Wei Li , Haixiao Di , Lihua Luo , Chunqi Zhu , Jie Yang , Xiaoyi Yin , Hang Yin , Jianqing Gao , Yongzhong Du , Jian You
|
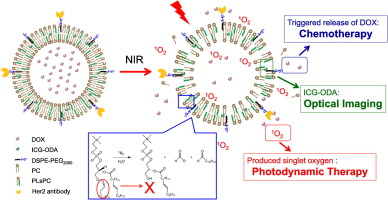
The targeted drug delivery with the help of nanocarriers and the controlled drug release at the lesion sites are the most effective ways to enhance therapeutic efficacy and reduce side effects. Here, we built a light sensitive liposome (Her2-I&D-LSL) which was formed by a special phospholipid (PLsPC) and a hydrophobically modified photosensitizer (ICG-ODA). DOX was employed as the therapeutic drug, encapsulating in the internal phase of the liposome whose surface was modified by Her2 antibodies for recognizing tumor cells with high Her2 receptor expression. Mediated by NIR light, Her2-I&D-LSL was proved to generate sufficient ROS to realize PDT, which then triggered the release of DOX for combined chemotherapy. The ROS generation and DOX release were verified to be strictly controlled by NIR light and the proportion of ICG-ODA. Thanks to the mediation of Her2 receptor, the specific DOX release and the combination of PDT-chemotherapy triggered by NIR light, Her2-I&D-LSL showed a significant accumulation in MCF7 and SKOV3 tumors, thus leading to the strongest tumor growth inhibition effect compared to PDT alone (I-LSL) or chemotherapy alone (D-LSL). Her2-I&D-LSL also possessed a great biocompatibility due to the targeted treatment, holding promise for future cancer therapy in clinic.
中文翻译:

具有近红外光的光敏脂质体触发了阿霉素的释放,作为光动力化学疗法的组合系统
借助于纳米载体的靶向药物递送和在病变部位的受控药物释放是增强治疗功效和减少副作用的最有效方法。在这里,我们建立了由特殊的磷脂(PLsPC)和疏水改性的光敏剂(ICG-ODA)形成的光敏脂质体(Her2-I&D-LSL)。DOX被用作治疗药物,包裹在脂质体的内相中,脂质体的表面被Her2抗体修饰,以识别具有高Her2受体表达的肿瘤细胞。在近红外光的作用下,Her2-I&D-LSL被证明可以产生足够的ROS来实现PDT,然后触发DOX的释放以进行联合化疗。ROS的产生和DOX的释放被证实是由NIR光和ICG-ODA的比例严格控制的。由于Her2受体的介导,特定的DOX释放以及近红外光触发的PDT化学疗法的结合,Her2-I&D-LSL在MCF7和SKOV3肿瘤中显示出大量蓄积,因此与之相比,具有最强的肿瘤生长抑制作用单独使用PDT(I-LSL)或单独使用化学疗法(D-LSL)。由于靶向治疗,Her2-I&D-LSL也具有很大的生物相容性,有望在临床上用于未来的癌症治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号