当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalysis of CO2 Electrochemical Reduction by Protonated Pyridine and Similar Molecules. Useful Lessons from a Methodological Misadventure
ACS Energy Letters ( IF 22.0 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1021/acsenergylett.8b00008 Cyrille Costentin 1 , Jean-Michel Savéant 1 , Cédric Tard 2
ACS Energy Letters ( IF 22.0 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1021/acsenergylett.8b00008 Cyrille Costentin 1 , Jean-Michel Savéant 1 , Cédric Tard 2
Affiliation
|
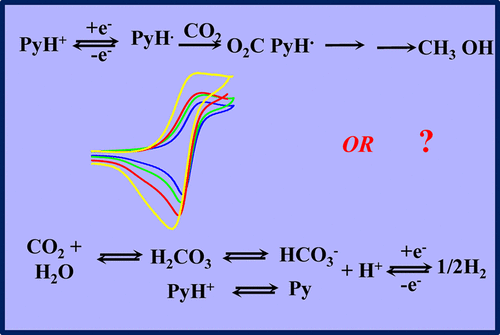
Electrochemical reduction of carbon dioxide is a particularly important issue in energy and environment contemporary challenges. It mobilized for several decades many researchers in the fields of catalysis by low-valent transition metal complexes. In this context, the report that a molecule as simple as protonated pyridine could catalyze the 6e– + 6H+ conversion of CO2 into methanol and triggered quite a lot of interest and amazement, even if the Faradaic yield was relatively modest (20%) and a precious metal (Pt) had to be used as an electrode material. Successive investigations of the same system or of systems involving molecules similar to protonated pyridine produced divergent results. The amount of data available at present from several research groups is sufficiently large to try to take stock of the situation. This concerns first and foremost a critical examination of the nature and Faradaic yields of electrolysis products. It also deals with the extensive use in this research of a nondestructive electrochemical technique—cyclic voltammetry—as a footprint of the existence of the catalysis process and a way to access its mechanism. As shown here, serious methodological problems arise in this connection, whose discussion may serve as general guidelines for safer future use of cyclic voltammetry. The same type of methodological questions also result from the theoretical computations that have abundantly accompanied experimental reports.
中文翻译:

质子化吡啶和类似分子催化CO 2电化学还原。方法论错误的有益教训
在当今能源和环境挑战中,二氧化碳的电化学还原是一个特别重要的问题。几十年来,它在低价过渡金属络合物催化领域动员了许多研究人员。在此背景下,有报道称像质子化吡啶这样的简单分子可以催化6e – + 6H + CO 2的转化。即使法拉第收率相对较低(20%),并且必须将贵金属(Pt)用作电极材料,也会引起很多兴趣和惊奇。对相同系统或涉及类似于质子化吡啶的分子的系统的连续研究产生了不同的结果。目前可以从几个研究小组获得的数据量足够大,可以用来估算这种情况。这首先涉及对电解产物的性质和法拉第收率的严格检查。在本研究中,它还涉及无损电化学技术(循环伏安法)的广泛使用,以此作为催化过程存在的足迹和获取其机理的途径。如此处所示,在这方面出现了严重的方法学问题,他的讨论可以作为将来更安全使用循环伏安法的一般指南。大量伴随实验报告的理论计算也产生了相同类型的方法学问题。
更新日期:2018-02-02
中文翻译:

质子化吡啶和类似分子催化CO 2电化学还原。方法论错误的有益教训
在当今能源和环境挑战中,二氧化碳的电化学还原是一个特别重要的问题。几十年来,它在低价过渡金属络合物催化领域动员了许多研究人员。在此背景下,有报道称像质子化吡啶这样的简单分子可以催化6e – + 6H + CO 2的转化。即使法拉第收率相对较低(20%),并且必须将贵金属(Pt)用作电极材料,也会引起很多兴趣和惊奇。对相同系统或涉及类似于质子化吡啶的分子的系统的连续研究产生了不同的结果。目前可以从几个研究小组获得的数据量足够大,可以用来估算这种情况。这首先涉及对电解产物的性质和法拉第收率的严格检查。在本研究中,它还涉及无损电化学技术(循环伏安法)的广泛使用,以此作为催化过程存在的足迹和获取其机理的途径。如此处所示,在这方面出现了严重的方法学问题,他的讨论可以作为将来更安全使用循环伏安法的一般指南。大量伴随实验报告的理论计算也产生了相同类型的方法学问题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号