当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isoindole Linkages Provide a Pathway for DOPAL-Mediated Cross-Linking of α-Synuclein.
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-15 , DOI: 10.1021/acs.biochem.7b01164 Jonathan W. Werner-Allen , Sarah Monti , Jenna F. DuMond , Rodney L. Levine , Ad Bax
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-15 , DOI: 10.1021/acs.biochem.7b01164 Jonathan W. Werner-Allen , Sarah Monti , Jenna F. DuMond , Rodney L. Levine , Ad Bax
|
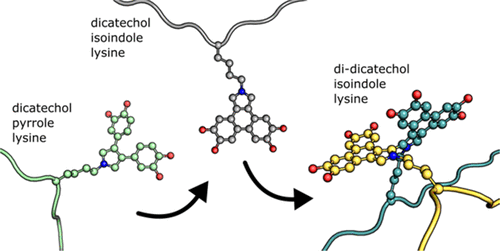
3,4-Dihydroxyphenylacetaldehyde (DOPAL) is a toxic and reactive product of dopamine catabolism. In the catecholaldehyde hypothesis for Parkinson's disease, it is a critical driver of the selective loss of dopaminergic neurons that characterizes the disease. DOPAL also cross-links α-synuclein, the main component of Lewy bodies, which are a pathological hallmark of the disease. We previously described the initial adduct formed in reactions between DOPAL and α-synuclein, a dicatechol pyrrole lysine (DCPL). Here, we examine the chemical basis for DOPAL-based cross-linking. We find that autoxidation of DCPL's catechol rings spurs its decomposition, yielding an intermediate dicatechol isoindole lysine (DCIL) product formed by an intramolecular reaction of the two catechol rings to give an unstable tetracyclic structure. DCIL then reacts with a second DCIL to give a dimeric, di-DCIL. This product is formed by an intermolecular carbon-carbon bond between the isoindole rings of the two DCILs that generates two structurally nonequivalent and separable atropisomers. Using α-synuclein, we demonstrate that the DOPAL-catalyzed formation of oligomers can be separated into two steps. The initial adduct formation occurs robustly within an hour, with DCPL as the main product, and the second step cross-links α-synuclein molecules. Exploiting this two-stage reaction, we use an isotopic labeling approach to show the predominant cross-linking mechanism is an interadduct reaction. Finally, we confirm that a mass consistent with a di-DCIL linkage can be observed in dimeric α-synuclein by mass spectrometry. Our work elucidates previously unknown pathways of catechol-based oxidative protein damage and will facilitate efforts to detect DOPAL-based cross-links in disease-state neurons.
中文翻译:

异吲哚连接为 DOPAL 介导的 α-突触核蛋白交联提供了途径。
3,4-二羟基苯乙醛 (DOPAL) 是多巴胺分解代谢的有毒反应产物。在帕金森病的儿茶酚醛假说中,它是多巴胺能神经元选择性丧失的关键驱动因素,而多巴胺能神经元是该疾病的特征。 DOPAL 还与 α-突触核蛋白交联,α-突触核蛋白是路易体的主要成分,是该疾病的病理标志。我们之前描述了 DOPAL 和 α-突触核蛋白(一种二酚吡咯赖氨酸 (DCPL))之间反应形成的初始加合物。在这里,我们研究了基于 DOPAL 的交联的化学基础。我们发现 DCPL 儿茶酚环的自氧化会刺激其分解,产生中间产物儿茶酚异吲哚赖氨酸 (DCIL),该产物是通过两个儿茶酚环的分子内反应形成的,从而形成不稳定的四环结构。然后 DCIL 与第二个 DCIL 反应生成二聚体 di-DCIL。该产品由两个 DCIL 的异吲哚环之间的分子间碳-碳键形成,产生两种结构不等价且可分离的阻转异构体。使用 α-突触核蛋白,我们证明 DOPAL 催化的寡聚体的形成可以分为两个步骤。初始加合物形成在一小时内剧烈发生,DCPL 为主要产物,第二步交联 α-突触核蛋白分子。利用这种两阶段反应,我们使用同位素标记方法来表明主要的交联机制是加合物反应。最后,我们确认通过质谱法可以在二聚体 α-突触核蛋白中观察到与二 DCIL 连接一致的质量。我们的工作阐明了以前未知的基于儿茶酚的氧化蛋白损伤的途径,并将有助于检测疾病状态神经元中基于 DOPAL 的交联。
更新日期:2018-02-02
中文翻译:

异吲哚连接为 DOPAL 介导的 α-突触核蛋白交联提供了途径。
3,4-二羟基苯乙醛 (DOPAL) 是多巴胺分解代谢的有毒反应产物。在帕金森病的儿茶酚醛假说中,它是多巴胺能神经元选择性丧失的关键驱动因素,而多巴胺能神经元是该疾病的特征。 DOPAL 还与 α-突触核蛋白交联,α-突触核蛋白是路易体的主要成分,是该疾病的病理标志。我们之前描述了 DOPAL 和 α-突触核蛋白(一种二酚吡咯赖氨酸 (DCPL))之间反应形成的初始加合物。在这里,我们研究了基于 DOPAL 的交联的化学基础。我们发现 DCPL 儿茶酚环的自氧化会刺激其分解,产生中间产物儿茶酚异吲哚赖氨酸 (DCIL),该产物是通过两个儿茶酚环的分子内反应形成的,从而形成不稳定的四环结构。然后 DCIL 与第二个 DCIL 反应生成二聚体 di-DCIL。该产品由两个 DCIL 的异吲哚环之间的分子间碳-碳键形成,产生两种结构不等价且可分离的阻转异构体。使用 α-突触核蛋白,我们证明 DOPAL 催化的寡聚体的形成可以分为两个步骤。初始加合物形成在一小时内剧烈发生,DCPL 为主要产物,第二步交联 α-突触核蛋白分子。利用这种两阶段反应,我们使用同位素标记方法来表明主要的交联机制是加合物反应。最后,我们确认通过质谱法可以在二聚体 α-突触核蛋白中观察到与二 DCIL 连接一致的质量。我们的工作阐明了以前未知的基于儿茶酚的氧化蛋白损伤的途径,并将有助于检测疾病状态神经元中基于 DOPAL 的交联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号