Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-02-02 , DOI: 10.1016/j.bioorg.2018.01.027 Monika Serafin-Lewańczuk , Magdalena Klimek-Ochab , Małgorzata Brzezińska-Rodak , Ewa Żymańczyk-Duda
|
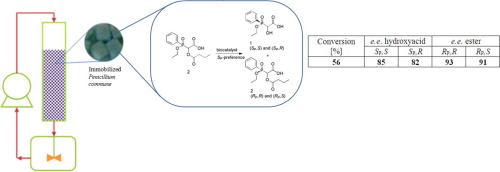
Chiral hydroxyphosphonates due to their wide range of biological properties are industrially important chemicals. Chemical synthesis of their optical isomers is expensive, time consuming and not friendly to the environment, so biotransformations are under consideration. Among others, these compounds act as enzymes inhibitors. This makes the bioconversions of phosphonates, especially scaling experiments, hard to perform. Biocatalysis is one of the methods that can be applied in synthesis of optically pure compounds. To increase the efficiency of the process with whole cell biocatalysts, it is essential to ensure optimal reaction conditions that minimize cellular stress and can enhance the metabolic activity of cells. The present investigation focuses on the scaling up of the kinetic resolution of racemic mixture of 2-butyryloxy-2-(ethoxy-P-phenylphosphinyl)acetic acid, applying free and immobilized form of the fungal biocatalysts and two operation systems: shake flask and recirculated fixed-bed batch reactor. Protocols of effective mycelium immobilization on polyurethane foams were set for T. purpurogenus IAFB 2512, F. oxysporum, P. commune. The best results of biotransformation were obtained with the immobilized P. commune in the column recirculated fixed-bed batch reactor. The conversion reaches 56% (maximal for the kinetic process) and the enantiomeric enrichment of the isomers mixture ranges between 82 and 93% (93% for ester of RP,R conformation). All biocatalysts exhibit SP-preference toward tested compound, what is essential because of importance of the phosphorus atom chirality for its biological activity.
中文翻译:

手性膦酸合成平台的真菌合成-方法的范围和局限性
手性羟基膦酸酯由于其广泛的生物学特性而成为工业上重要的化学品。它们的旋光异构体的化学合成昂贵,费时且对环境不友好,因此正在考虑生物转化。这些化合物尤其充当酶抑制剂。这使得很难进行膦酸酯的生物转化,尤其是定标实验。生物催化是可用于合成光学纯化合物的方法之一。为了提高使用全细胞生物催化剂的过程的效率,至关重要的是要确保最佳的反应条件,以最小化细胞应激并增强细胞的代谢活性。本研究着重于扩大2-丁酰氧基-2-(乙氧基-P-苯基次膦基)乙酸的外消旋混合物的动力学拆分,应用游离和固定形式的真菌生物催化剂和两种操作系统:摇瓶和再循环固定床间歇式反应器。制定了有效的将菌丝体固定在聚氨酯泡沫上的方案T.purpurogenogen IAFB 2512 , F.oxysporum ,P。公社。在柱循环固定床间歇式反应器中,固定化的P. com社获得了最佳的生物转化结果。转化率达到56%(对于动力学过程是最大的),并且异构体混合物的对映异构体富集在82%和93%之间(对于R P,R构象的酯为93%)。所有生物催化剂均表现出对被测化合物的S P优先,这是必不可少的,因为磷原子手性对其生物活性至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号