当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of β‐Naphthols and Naphthofuranones from ortho‐Alkynylarylketones via Sequential AgTFA‐Catalyzed Ketonization–Intramolecular Aldol Condensation: A Total Synthesis of Negundin A
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-03-13 , DOI: 10.1002/ajoc.201800025 Phongprapan Nimnual 1 , Krissada Norseeda 1 , Bhornrawin Akkachairin 1 , Jumreang Tummatorn 1, 2 , Pavitra Laohapaisan 1 , Nantamon Supantanapong 1 , Pennapa Chuangsoongnern 2 , Charnsak Thongsornkleeb 1, 3 , Satapanawat Sittihan 1 , Somsak Ruchirawat 1, 2 , Warabhorn Rodphon 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-03-13 , DOI: 10.1002/ajoc.201800025 Phongprapan Nimnual 1 , Krissada Norseeda 1 , Bhornrawin Akkachairin 1 , Jumreang Tummatorn 1, 2 , Pavitra Laohapaisan 1 , Nantamon Supantanapong 1 , Pennapa Chuangsoongnern 2 , Charnsak Thongsornkleeb 1, 3 , Satapanawat Sittihan 1 , Somsak Ruchirawat 1, 2 , Warabhorn Rodphon 2
Affiliation
|
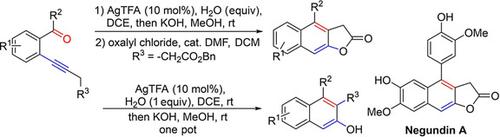
A sequential synthesis of β‐naphthols and naphtho[2,3‐b]furan‐2(3H)‐ones (naphthofuranones) has been developed using ortho‐alkynylarylketones as the substrates. The AgTFA‐catalyzed ketonization generated the corresponding 1,5‐diketone intermediates in situ; these were further cyclized via aldol condensation under basic conditions to obtain β‐naphthol products in moderate to good yields. For the synthesis of naphtho[2,3‐b]furan‐2(3H)‐ones, an additional lactonization step was applied to give a broad range of products in good yields. Moreover, this method has also been utilized in the synthesis of natural product negundin A.
中文翻译:

通过顺序AgTFA催化酮化-分子内羟醛缩合反应由邻炔基芳基酮合成β-萘酚和萘呋喃酮:Negundin A的总合成
已开发了以邻炔基芳基酮为底物的β-萘酚和萘[2,3 - b ]呋喃-2(3 H)-酮(萘呋喃酮)的顺序合成方法。AgTFA催化的酮化反应可在原位生成相应的1,5-二酮中间体。这些在碱性条件下通过羟醛缩合进一步环化,以中等至良好的收率获得β-萘酚产品。为了合成萘并[2,3 - b ]呋喃-2(3 H)-酮,采用了额外的内酯化步骤,从而以高收率获得了种类繁多的产品。此外,该方法也已用于合成天然产物negundinA。
更新日期:2018-03-13
中文翻译:

通过顺序AgTFA催化酮化-分子内羟醛缩合反应由邻炔基芳基酮合成β-萘酚和萘呋喃酮:Negundin A的总合成
已开发了以邻炔基芳基酮为底物的β-萘酚和萘[2,3 - b ]呋喃-2(3 H)-酮(萘呋喃酮)的顺序合成方法。AgTFA催化的酮化反应可在原位生成相应的1,5-二酮中间体。这些在碱性条件下通过羟醛缩合进一步环化,以中等至良好的收率获得β-萘酚产品。为了合成萘并[2,3 - b ]呋喃-2(3 H)-酮,采用了额外的内酯化步骤,从而以高收率获得了种类繁多的产品。此外,该方法也已用于合成天然产物negundinA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号