当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zn(II) Byproduct Enhances the Cu-Catalyzed Cross-Coupling of Bromozinc Difluorophosphonate with Iodobenzoates: A DFT Study
Organometallics ( IF 2.5 ) Pub Date : 2018-02-01 00:00:00 , DOI: 10.1021/acs.organomet.7b00739 Jesús Jover 1, 2
Organometallics ( IF 2.5 ) Pub Date : 2018-02-01 00:00:00 , DOI: 10.1021/acs.organomet.7b00739 Jesús Jover 1, 2
Affiliation
|
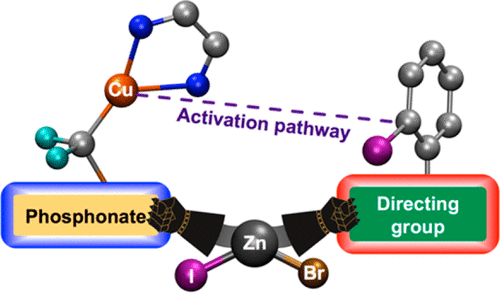
The copper-catalyzed cross-coupling of bromozinc difluorophosphonate with iodobenzoates has been studied with the DFT methodology in order to understand the experimentally observed reactivity. The directing carboxylate group promotes the reaction for methyl 2-iodobenzoate and, unexpectedly, also for methyl 4-iodobenzoate, although to a lesser extent. DFT calculations show that the Zn(II) byproduct, formed in the initial stages of the reaction, remains attached to the catalyst and serves as an anchoring point for the benzoate moiety, allowing in turn the reaction for both ortho- and para-substituted iodobenzoates. The computationally derived reaction mechanism has also been applied to study whether other substrates may engage in a similar cross-coupling process with bromozinc difluorophosphonate. The calculations carried out on substrates bearing nitrogen-directing groups, such as triazene and pyridine, indicate that their reactions should be possible and that the latter should produce a much faster reaction in comparison to iodobenzoates.
中文翻译:

锌(II)副产物增强铜催化的二氟膦酸溴锌与碘代苯甲酸酯的交叉偶联:DFT研究
为了理解实验观察到的反应性,已经用DFT方法研究了溴化二氟膦酸溴锌与碘代苯甲酸酯的铜催化交叉偶联。指导的羧酸根基团促进了2-碘代苯甲酸甲酯的反应,并且出乎意料地也促进了4-碘代苯甲酸甲酯的反应,尽管程度较小。DFT计算表明,在反应初始阶段形成的Zn(II)副产物保持附着在催化剂上,并充当苯甲酸酯部分的锚定点,从而允许邻位和对位取代的碘代苯甲酸酯反应。通过计算得出的反应机理也已被用于研究其他底物是否可以与溴氟锌二氟膦酸酯进行类似的交叉偶联过程。
更新日期:2018-02-02
中文翻译:

锌(II)副产物增强铜催化的二氟膦酸溴锌与碘代苯甲酸酯的交叉偶联:DFT研究
为了理解实验观察到的反应性,已经用DFT方法研究了溴化二氟膦酸溴锌与碘代苯甲酸酯的铜催化交叉偶联。指导的羧酸根基团促进了2-碘代苯甲酸甲酯的反应,并且出乎意料地也促进了4-碘代苯甲酸甲酯的反应,尽管程度较小。DFT计算表明,在反应初始阶段形成的Zn(II)副产物保持附着在催化剂上,并充当苯甲酸酯部分的锚定点,从而允许邻位和对位取代的碘代苯甲酸酯反应。通过计算得出的反应机理也已被用于研究其他底物是否可以与溴氟锌二氟膦酸酯进行类似的交叉偶联过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号