Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2018-01-31 , DOI: 10.1016/j.jaci.2017.12.984 Johanna Sophie Alfen , Paola Larghi , Federica Facciotti , Nicola Gagliani , Roberto Bosotti , Moira Paroni , Stefano Maglie , Paola Gruarin , Chiara Maria Vasco , Valeria Ranzani , Cristina Frusteri , Andrea Iseppon , Monica Moro , Maria Cristina Crosti , Stefano Gatti , Massimiliano Pagani , Flavio Caprioli , Sergio Abrignani , Richard A. Flavell , Jens Geginat
|
Background
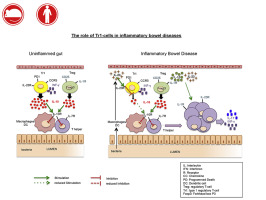
IL-10 is an anti-inflammatory cytokine required for intestinal immune homeostasis. It mediates suppression of T-cell responses by type 1 regulatory T (TR1) cells but is also produced by CD25+ regulatory T (Treg) cells.
Objective
We aimed to identify and characterize human intestinal TR1 cells and to investigate whether they are a relevant cellular source of IL-10 in patients with inflammatory bowel diseases (IBDs).
Methods
CD4+ T cells isolated from the intestinal lamina propria of human subjects and mice were analyzed for phenotype, cytokine production, and suppressive capacities. Intracellular IL-10 expression by CD4+ T-cell subsets in the inflamed guts of patients with IBD (Crohn disease or ulcerative colitis) was compared with that in cells from noninflamed control subjects. Finally, the effects of proinflammatory cytokines on T-cell IL-10 expression were analyzed, and IL-1β and IL-23 responsiveness was assessed.
Results
Intestinal TR1 cells could be identified by coexpression of CCR5 and programmed cell death protein 1 (PD-1) in human subjects and mice. CCR5+PD-1+ TR1 cells expressed IFN-γ and efficiently suppressed T-cell proliferation and transfer colitis. Intestinal IFN-γ+ TR1 cells, but not IL-7 receptor–positive TH cells or CD25+ Treg cells, showed lower IL-10 expression in patients with IBDs. TR1 cells were responsive to IL-23, and IFN-γ+ TR1 cells downregulated IL-10 with IL-1β and IL-23. Conversely, CD25+ Treg cells expressed higher levels of IL-1 receptor but showed stable IL-10 expression.
Conclusions
We provide the first ex vivo characterization of human intestinal TR1 cells. Selective downregulation of IL-10 by IFN-γ+ TR1 cells in response to proinflammatory cytokines is likely to drive excessive intestinal inflammation in patients with IBDs.











































 京公网安备 11010802027423号
京公网安备 11010802027423号