当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Preparation of a Key Hydroxylamine Intermediate for Relebactam: Rate Enhancement of Benzyl Ether Hydrogenolysis with DABCO
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-02-01 00:00:00 , DOI: 10.1021/acs.oprd.7b00381 Jianguo Yin 1 , Mark Weisel 1 , Yining Ji 1 , Zhijian Liu 1 , Jinchu Liu 1 , Debra J. Wallace 1 , Feng Xu 1 , Benjamin D. Sherry 1 , Nobuyoshi Yasuda 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-02-01 00:00:00 , DOI: 10.1021/acs.oprd.7b00381 Jianguo Yin 1 , Mark Weisel 1 , Yining Ji 1 , Zhijian Liu 1 , Jinchu Liu 1 , Debra J. Wallace 1 , Feng Xu 1 , Benjamin D. Sherry 1 , Nobuyoshi Yasuda 1
Affiliation
|
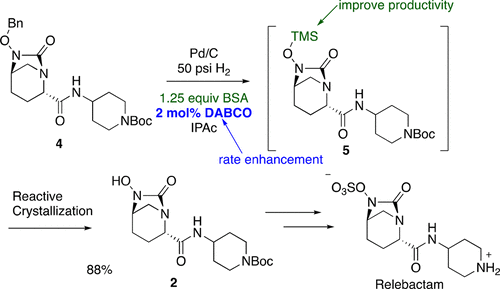
Previous methods to prepare a bicyclic N-hydroxyl urea intermediate in the synthesis of the potent β-lactamase inhibitor relebactam were effective, but deemed unsuitable for long-term use. Therefore, we developed an in situ protection protocol during hydrogenolysis and a robust deprotection/isolation sequence of this unstable intermediate employing a reactive crystallization. During the hydrogenation studies, we discovered a significant rate enhancement of O-benzyl ether hydrogenolysis in the presence of organic amine bases, especially DABCO. The broader utility of the application of organic bases on the hydrogenolysis of a range of O- and N-benzyl-containing substrates was demonstrated.
中文翻译:

改善雷贝巴坦关键羟胺中间体的制备:用DABCO加速苄醚氢解的速率
在有效的β-内酰胺酶抑制剂雷巴坦的合成中,制备双环N-羟基脲中间体的先前方法是有效的,但认为不适合长期使用。因此,我们开发了氢解过程中的原位保护方案,并采用反应性结晶技术对该不稳定中间体进行了稳健的脱保护/分离步骤。在氢化研究期间,我们发现在有机胺碱(尤其是DABCO)存在下,O-苄基醚氢解反应的速率显着提高。证明了在一系列含O-和N-苄基的底物进行氢解后,有机碱的应用具有更广泛的用途。
更新日期:2018-02-01
中文翻译:

改善雷贝巴坦关键羟胺中间体的制备:用DABCO加速苄醚氢解的速率
在有效的β-内酰胺酶抑制剂雷巴坦的合成中,制备双环N-羟基脲中间体的先前方法是有效的,但认为不适合长期使用。因此,我们开发了氢解过程中的原位保护方案,并采用反应性结晶技术对该不稳定中间体进行了稳健的脱保护/分离步骤。在氢化研究期间,我们发现在有机胺碱(尤其是DABCO)存在下,O-苄基醚氢解反应的速率显着提高。证明了在一系列含O-和N-苄基的底物进行氢解后,有机碱的应用具有更广泛的用途。









































 京公网安备 11010802027423号
京公网安备 11010802027423号