当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleophilic difluoromethylation of aromatic aldehydes using trimethyl(trifluoromethyl)silane (TMSCF3)
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-01-31 , DOI: 10.1016/j.jfluchem.2018.01.009 Sankarganesh Krishnamoorthy , Sayan Kar , Jotheeswari Kothandaraman , G.K. Surya Prakash
中文翻译:

使用三甲基(三氟甲基)硅烷(TMSCF 3)进行芳香族醛的亲核二氟甲基化
更新日期:2018-01-31
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-01-31 , DOI: 10.1016/j.jfluchem.2018.01.009 Sankarganesh Krishnamoorthy , Sayan Kar , Jotheeswari Kothandaraman , G.K. Surya Prakash
|
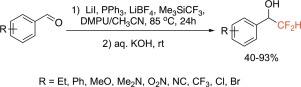
Reaction of the Ruppert–Prakash reagent (Me3SiCF3) with aromatic aldehydes in the presence of triphenylphosphine, lithium iodide and lithium tetrafluoroborate selectively furnishes gem-difluorinated phosphonium salts. Simple alkaline hydrolysis of these salts results in difluoromethylated products. Thus, one-pot nucleophilic difluoromethylation of aromatic aldehydes using Me3SiCF3 has been accomplished. The protocol tolerates electron withdrawing as well as electron donating substituents.
中文翻译:

使用三甲基(三氟甲基)硅烷(TMSCF 3)进行芳香族醛的亲核二氟甲基化
在三苯基膦,碘化锂和四氟硼酸锂的存在下,Ruppert-Prakash试剂(Me 3 SiCF 3)与芳族醛的反应选择性地提供了宝石二氟化phospho盐。这些盐的简单碱性水解产生二氟甲基化产物。因此,已经实现了使用Me 3 SiCF 3一锅法芳香族醛的亲核二氟甲基化。该方案容许吸电子以及给电子取代基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号