当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel class of human 15‐LOX‐1 inhibitors based on 3‐hydroxycoumarin
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-02-26 , DOI: 10.1111/cbdd.13174 Seyed Jamal Alavi 1 , Hamid Sadeghian 2, 3 , Seyed Mohammad Seyedi 1 , Alireza Salimi 1 , Hossein Eshghi 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-02-26 , DOI: 10.1111/cbdd.13174 Seyed Jamal Alavi 1 , Hamid Sadeghian 2, 3 , Seyed Mohammad Seyedi 1 , Alireza Salimi 1 , Hossein Eshghi 1
Affiliation
|
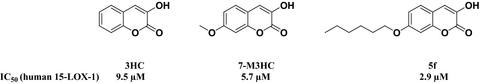
Inflammations, sensitivities, and some cancers in mammals are intimately linked to the activity of lipo‐oxygenase enzymes. Owing to the importance of these enzymes, mechanistic studies, product analysis, and synthesis of inhibitors have expanded. In this study, a series of hydroxycoumarins, methoxy‐3‐hydroxy coumarins, and 7‐alkoxy‐3‐hydroxy coumarins were synthesized and evaluated as potential inhibitors of human 15‐LOX‐1. Among the synthetic coumarins, 7‐methoxy‐3‐hydroxycoumarin derivative demonstrated potent inhibitory activity and the compound, 5f, showed the best result. Radical scavenging assessment, IC50, HNMR, and DPPH bleaching results indicate that the electronic properties are the major factors for the lipo‐oxygenase inhibition potency of the synthetic coumarins. Based on the theoretical studies, it was suggested that the mesomeric effect of the substituent at the seventh position of the benzene ring is one of the major factors in the stability of the oxy‐radical intermediate.
中文翻译:

基于3-羟基香豆素的新型人类15-LOX-1抑制剂
哺乳动物的炎症,敏感性和某些癌症与脂氧合酶的活性密切相关。由于这些酶的重要性,机理研究,产物分析和抑制剂的合成得到了扩展。在这项研究中,合成了一系列羟基香豆素,甲氧基-3-羟基香豆素和7-烷氧基-3-羟基香豆素,并将其评估为人类15-LOX-1的潜在抑制剂。在合成香豆素中,7-甲氧基-3-羟基香豆素衍生物显示出有效的抑制活性,化合物5f显示出最佳的抑制效果。自由基清除评估,IC 50,HNMR和DPPH漂白结果表明,电子性能是合成香豆素抑制脂加氧酶效能的主要因素。根据理论研究,有人提出苯环第七位取代基的介观效应是氧自由基中间体稳定性的主要因素之一。
更新日期:2018-02-26
中文翻译:

基于3-羟基香豆素的新型人类15-LOX-1抑制剂
哺乳动物的炎症,敏感性和某些癌症与脂氧合酶的活性密切相关。由于这些酶的重要性,机理研究,产物分析和抑制剂的合成得到了扩展。在这项研究中,合成了一系列羟基香豆素,甲氧基-3-羟基香豆素和7-烷氧基-3-羟基香豆素,并将其评估为人类15-LOX-1的潜在抑制剂。在合成香豆素中,7-甲氧基-3-羟基香豆素衍生物显示出有效的抑制活性,化合物5f显示出最佳的抑制效果。自由基清除评估,IC 50,HNMR和DPPH漂白结果表明,电子性能是合成香豆素抑制脂加氧酶效能的主要因素。根据理论研究,有人提出苯环第七位取代基的介观效应是氧自由基中间体稳定性的主要因素之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号