当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
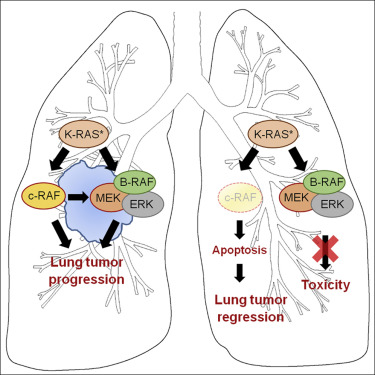
c-RAF Ablation Induces Regression of Advanced Kras/Trp53 Mutant Lung Adenocarcinomas by a Mechanism Independent of MAPK Signaling.
Cancer Cell ( IF 50.3 ) Pub Date : 2018-02-12 , DOI: 10.1016/j.ccell.2017.12.014 Manuel Sanclemente , Sarah Francoz , Laura Esteban-Burgos , Emilie Bousquet-Mur , Magdolna Djurec , Pedro P. Lopez-Casas , Manuel Hidalgo , Carmen Guerra , Matthias Drosten , Monica Musteanu , Mariano Barbacid
Cancer Cell ( IF 50.3 ) Pub Date : 2018-02-12 , DOI: 10.1016/j.ccell.2017.12.014 Manuel Sanclemente , Sarah Francoz , Laura Esteban-Burgos , Emilie Bousquet-Mur , Magdolna Djurec , Pedro P. Lopez-Casas , Manuel Hidalgo , Carmen Guerra , Matthias Drosten , Monica Musteanu , Mariano Barbacid
|
A quarter of all solid tumors harbor KRAS oncogenes. Yet, no selective drugs have been approved to treat these malignancies. Genetic interrogation of the MAPK pathway revealed that systemic ablation of MEK or ERK kinases in adult mice prevent tumor development but are unacceptably toxic. Here, we demonstrate that ablation of c-RAF expression in advanced tumors driven by KrasG12V/Trp53 mutations leads to significant tumor regression with no detectable appearance of resistance mechanisms. Tumor regression results from massive apoptosis. Importantly, systemic abrogation of c-RAF expression does not inhibit canonical MAPK signaling, hence, resulting in limited toxicities. These results are of significant relevance for the design of therapeutic strategies to treat K-RAS mutant cancers.
中文翻译:

c-RAF切除通过独立于MAPK信号传导的机制诱导晚期Kras / Trp53突变型肺腺癌的消退。
所有实体瘤的四分之一都带有KRAS癌基因。然而,尚未批准选择性药物治疗这些恶性肿瘤。对MAPK途径的遗传询问显示,成年小鼠全身消融MEK或ERK激酶可阻止肿瘤发展,但毒性不可接受。在这里,我们证明了在由Kras G12V / Trp53突变驱动的晚期肿瘤中c-RAF表达的消融导致明显的肿瘤消退,而没有可检测到的耐药机制。大量细胞凋亡导致肿瘤消退。重要的是,全身废除c-RAF表达不会抑制规范性MAPK信号传导,因此,导致毒性有限。这些结果对于设计治疗K-RAS突变型癌症的治疗策略具有重要的意义。
更新日期:2018-01-31
中文翻译:

c-RAF切除通过独立于MAPK信号传导的机制诱导晚期Kras / Trp53突变型肺腺癌的消退。
所有实体瘤的四分之一都带有KRAS癌基因。然而,尚未批准选择性药物治疗这些恶性肿瘤。对MAPK途径的遗传询问显示,成年小鼠全身消融MEK或ERK激酶可阻止肿瘤发展,但毒性不可接受。在这里,我们证明了在由Kras G12V / Trp53突变驱动的晚期肿瘤中c-RAF表达的消融导致明显的肿瘤消退,而没有可检测到的耐药机制。大量细胞凋亡导致肿瘤消退。重要的是,全身废除c-RAF表达不会抑制规范性MAPK信号传导,因此,导致毒性有限。这些结果对于设计治疗K-RAS突变型癌症的治疗策略具有重要的意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号