当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DFT Studies on the Water-Assisted Synergistic Proton Dissociation Mechanism for the Spontaneous Hydrolysis Reaction of Al3+ in Aqueous Solution
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00142 Shaonan Dong 1 , Wenjing Shi 1 , Jing Zhang 1 , Shuping Bi 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00142 Shaonan Dong 1 , Wenjing Shi 1 , Jing Zhang 1 , Shuping Bi 1
Affiliation
|
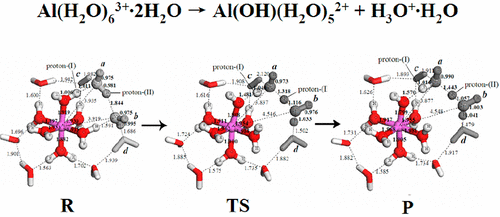
The kinetic mechanism of spontaneous aluminum ion (Al3+) hydrolysis reaction in aqueous solution is investigated using the density functional theory–quantum chemical cluster model method. Three typical reaction pathways for the spontaneous Al3+ hydrolysis reaction are modeled, including (1) the traditional spontaneous proton dissociation on the Al3+ inner-shell coordinated waters; (2) the conventional bulk water-assisted proton dissociation; and (3) the second-shell water-assisted synergistic dissociation of the protons on the Al3+ inner-shell waters. The results show that the electrostatic effects between Al3+ and its coordinated waters alone cannot fully account for the proton loss on an inner-shell coordinated water. It is suggested that the main reaction pathway for natural hydrolysis of aqueous Al3+ is the second-shell water-assisted synergistic proton dissociation, in which the participation of the second hydration shell is crucially important. The calculated synergistic proton dissociation rate constant, kH+ = 1.14 × 105 s–1, is in close agreement with the experimental results (1.09 × 105 s–1 and 7.9 × 104 s–1). The first hydrolysis equilibrium constant pKa1 of Al3+ is calculated as 5.82, also consistent with the literature value of 5.00. This work elucidates the molecular mechanism of the spontaneous Al3+ hydrolysis reaction in natural waters and has important environmental implications.
中文翻译:

水溶液中Al 3+自发水解反应的水辅助协同质子解离机理的DFT研究
利用密度泛函理论-量子化学簇模型方法研究了水溶液中自发铝离子(Al 3+)水解反应的动力学机理。模拟了自发Al 3+水解反应的三种典型反应途径,包括:(1)传统的Al 3+内壳配位水上的自发质子解离;(2)常规的大体积水辅助质子解离;(3)质子在Al 3+内壳水上的第二层水辅助协同解离。结果表明,Al 3+之间的静电效应而且仅靠其配水就不能完全解决内壳配水上的质子损失。有人认为,Al 3+水溶液自然水解的主要反应途径是第二壳水辅助的协同质子解离,其中第二水合壳的参与至关重要。计算出的协同质子解离速率常数k H + = 1.14×10 5 s –1与实验结果(1.09×10 5 s –1和7.9×10 4 s –1)非常吻合。Al的第一水解平衡常数p K a1计算出的3+为5.82,也与文献值5.00一致。这项工作阐明了自然水中自发的Al 3+水解反应的分子机理,并具有重要的环境意义。
更新日期:2018-01-29
中文翻译:

水溶液中Al 3+自发水解反应的水辅助协同质子解离机理的DFT研究
利用密度泛函理论-量子化学簇模型方法研究了水溶液中自发铝离子(Al 3+)水解反应的动力学机理。模拟了自发Al 3+水解反应的三种典型反应途径,包括:(1)传统的Al 3+内壳配位水上的自发质子解离;(2)常规的大体积水辅助质子解离;(3)质子在Al 3+内壳水上的第二层水辅助协同解离。结果表明,Al 3+之间的静电效应而且仅靠其配水就不能完全解决内壳配水上的质子损失。有人认为,Al 3+水溶液自然水解的主要反应途径是第二壳水辅助的协同质子解离,其中第二水合壳的参与至关重要。计算出的协同质子解离速率常数k H + = 1.14×10 5 s –1与实验结果(1.09×10 5 s –1和7.9×10 4 s –1)非常吻合。Al的第一水解平衡常数p K a1计算出的3+为5.82,也与文献值5.00一致。这项工作阐明了自然水中自发的Al 3+水解反应的分子机理,并具有重要的环境意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号