当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Islet Amyloid Polypeptide Promotes Amyloid-Beta Aggregation by Binding-Induced Helix-Unfolding of the Amyloidogenic Core
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1021/acschemneuro.7b00396 Xinwei Ge , Ye Yang , Yunxiang Sun , Weiguo Cao , Feng Ding
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1021/acschemneuro.7b00396 Xinwei Ge , Ye Yang , Yunxiang Sun , Weiguo Cao , Feng Ding
|
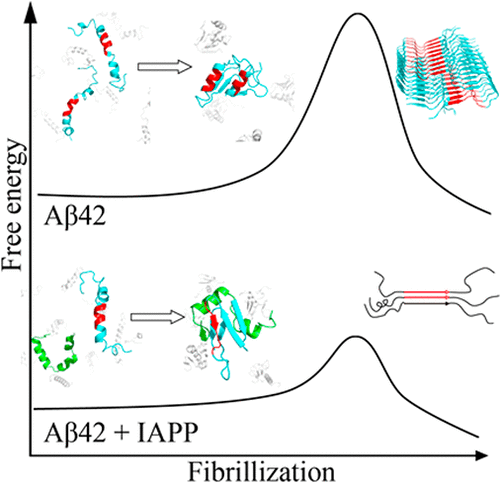
Amyloid aggregation of amyloid-beta (Aβ) and islet amyloid polypeptide (IAPP) is associated with Alzheimer’s disease (AD) and type-2 diabetes (T2D), respectively. With T2D being the risk factor for AD and the ability of IAPP to cross the blood-brain barrier, the coaggregation of Aβ and IAPP has been explored to understand the cross-talk between the two diseases. Recent studies demonstrated that soluble IAPP could significantly accelerate the aggregation of Aβ while preformed amyloids of IAPP were poor “seeds” for Aβ aggregation. Here, we apply all-atom discrete molecular dynamics simulations to investigate possible molecular mechanisms for the accelerated coaggregation of IAPP and Aβ42 comparing to Aβ42 aggregation alone, which was confirmed by the complementary thioflavin-T fluorescence assay. Our simulation results suggest that peptides in the mixture tend to form heterodimers as the first step toward their coaggregation. Strong interpeptide interactions with IAPP in the heterodimer shift the helical conformation of Aβ42 in its amyloidogenic central hydrophobic core, residues 16–22 (Aβ16–22), to the extended conformation ready to form β-sheets. Our study suggests that the unfolding of Aβ16–22 helix contributes to the aggregation free-energy barrier and corresponds to the rate-limiting conformational change for Aβ42 aggregation. Therefore, we propose that soluble IAPP promotes the aggregation of Aβ42 by binding-induced conformational change of Aβ42 in its amyloidogenic core and thus reduced aggregation free-energy barrier.
中文翻译:

胰岛淀粉样多肽通过淀粉样生成核心的结合诱导的螺旋展开促进淀粉样-β聚集。
淀粉样蛋白β(Aβ)和胰岛淀粉样蛋白多肽(IAPP)的淀粉样蛋白聚集分别与阿尔茨海默氏病(AD)和2型糖尿病(T2D)相关。由于T2D是AD的危险因素,并且IAPP能够穿越血脑屏障,因此已经探索了Aβ和IAPP的聚集,以了解两种疾病之间的相互影响。最近的研究表明,可溶性IAPP可以显着加速Aβ的聚集,而预先形成的IAPP淀粉样蛋白则是Aβ聚集的“种子”。在这里,我们应用全原子离散分子动力学模拟来研究与单独的Aβ42聚集相比,IAPP和Aβ42的加速共聚集的可能分子机制,这通过互补的硫代黄素-T荧光测定法得到了证实。我们的模拟结果表明,混合物中的肽倾向于形成异二聚体,这是它们共聚的第一步。在异二聚体中与IAPP的强肽间相互作用将Aβ42的淀粉样蛋白中心疏水核心(残基16–22(Aβ16–22))中的螺旋构象转变为易于形成β-折叠的扩展构象。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。在异二聚体中与IAPP的强肽间相互作用将Aβ42的淀粉样蛋白中心疏水核心(残基16–22(Aβ16–22))中的螺旋构象转变为易于形成β-折叠的扩展构象。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。在异二聚体中与IAPP的强肽间相互作用将Aβ42的淀粉样蛋白中心疏水核心(残基16–22(Aβ16–22))中的螺旋构象转变为易于形成β-折叠的扩展构象。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出可溶性IAPP通过在其淀粉样生成核心中结合诱导的Aβ42构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。
更新日期:2018-01-29
中文翻译:

胰岛淀粉样多肽通过淀粉样生成核心的结合诱导的螺旋展开促进淀粉样-β聚集。
淀粉样蛋白β(Aβ)和胰岛淀粉样蛋白多肽(IAPP)的淀粉样蛋白聚集分别与阿尔茨海默氏病(AD)和2型糖尿病(T2D)相关。由于T2D是AD的危险因素,并且IAPP能够穿越血脑屏障,因此已经探索了Aβ和IAPP的聚集,以了解两种疾病之间的相互影响。最近的研究表明,可溶性IAPP可以显着加速Aβ的聚集,而预先形成的IAPP淀粉样蛋白则是Aβ聚集的“种子”。在这里,我们应用全原子离散分子动力学模拟来研究与单独的Aβ42聚集相比,IAPP和Aβ42的加速共聚集的可能分子机制,这通过互补的硫代黄素-T荧光测定法得到了证实。我们的模拟结果表明,混合物中的肽倾向于形成异二聚体,这是它们共聚的第一步。在异二聚体中与IAPP的强肽间相互作用将Aβ42的淀粉样蛋白中心疏水核心(残基16–22(Aβ16–22))中的螺旋构象转变为易于形成β-折叠的扩展构象。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。在异二聚体中与IAPP的强肽间相互作用将Aβ42的淀粉样蛋白中心疏水核心(残基16–22(Aβ16–22))中的螺旋构象转变为易于形成β-折叠的扩展构象。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。在异二聚体中与IAPP的强肽间相互作用将Aβ42的淀粉样蛋白中心疏水核心(残基16–22(Aβ16–22))中的螺旋构象转变为易于形成β-折叠的扩展构象。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出可溶性IAPP通过在其淀粉样生成核心中结合诱导的Aβ42构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。我们的研究表明,Aβ16-22螺旋的展开有助于聚集自由能屏障,并对应于Aβ42聚集的限速构象变化。因此,我们提出,可溶性IAPP通过结合诱导淀粉样生成核心中Aβ42的构象变化来促进Aβ42的聚集,从而降低聚集自由能屏障。











































 京公网安备 11010802027423号
京公网安备 11010802027423号