当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cathodic Corrosion of Zinc under Potentiostatic Conditions in NaCl Solutions
ChemElectroChem ( IF 4 ) Pub Date : 2018-02-15 , DOI: 10.1002/celc.201701325 Michel Prestat 1 , Josiane Soares Costa 1, 2 , Benoit Lescop 2 , Stéphane Rioual 2 , Lorenz Holzer 3 , Dominique Thierry 1
ChemElectroChem ( IF 4 ) Pub Date : 2018-02-15 , DOI: 10.1002/celc.201701325 Michel Prestat 1 , Josiane Soares Costa 1, 2 , Benoit Lescop 2 , Stéphane Rioual 2 , Lorenz Holzer 3 , Dominique Thierry 1
Affiliation
|
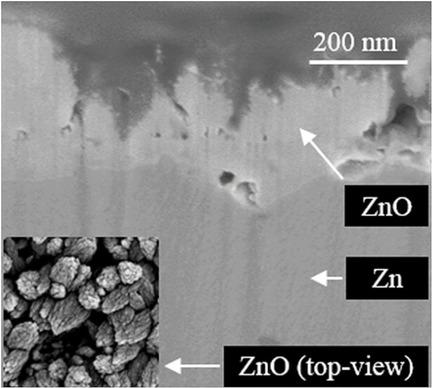
Zinc electrodes were polarized cathodically at moderate overpotentials in NaCl 0.6 M solutions under potentiostatic conditions for 7 to 17 hours at room temperature. Corrosion products were characterized by using optical microscopy, XRD, Raman microscopy, XPS, and FIB‐SEM. Close to the open‐circuit potential, the corrosion products were formed by simonkolleite and the electrochemical response exhibits anodic features. At more negative potentials, the current density remains cathodic throughout the polarization and the deposits on the electrode surface consist almost solely of ZnO. The soluble zinc species necessary for ZnO deposition originate from localized dissolution of the substrate in the form of pits. This effect is assigned to the strong alkalinization of the surface due to oxygen reduction. Despite developing greater surface area than bare zinc substrates, the nanostructured ZnO deposits reduced the cathodic activity.
中文翻译:

恒电位条件下NaCl溶液中锌的阴极腐蚀
锌电极在恒电位条件下,在NaCl 0.6 M溶液中的中等过电势下,在室温下阴极极化7至17小时。使用光学显微镜,XRD,拉曼显微镜,XPS和FIB-SEM对腐蚀产物进行表征。接近开路电势时,腐蚀产物是由次硅藻土形成的,电化学反应具有阳极特征。在更多的负电势下,整个极化过程中电流密度保持阴极状态,电极表面上的沉积物几乎仅由ZnO组成。ZnO沉积所需的可溶性锌物质源自基坑以点状形式的局部溶解。该作用归因于由于氧还原而引起的表面的强碱化。
更新日期:2018-02-15
中文翻译:

恒电位条件下NaCl溶液中锌的阴极腐蚀
锌电极在恒电位条件下,在NaCl 0.6 M溶液中的中等过电势下,在室温下阴极极化7至17小时。使用光学显微镜,XRD,拉曼显微镜,XPS和FIB-SEM对腐蚀产物进行表征。接近开路电势时,腐蚀产物是由次硅藻土形成的,电化学反应具有阳极特征。在更多的负电势下,整个极化过程中电流密度保持阴极状态,电极表面上的沉积物几乎仅由ZnO组成。ZnO沉积所需的可溶性锌物质源自基坑以点状形式的局部溶解。该作用归因于由于氧还原而引起的表面的强碱化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号