当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonanuclear Ni(ii) complexes in a [1-7-1] formation derived from asymmetric multidentate ligands: magnetic and electrochemical properties†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1039/c8dt00161h Yasuhiro Tsuji 1, 2, 3, 4, 5 , Tatsuo Togo 1, 2, 3, 4, 5 , Akio Mishima 1, 2, 3, 4, 5 , Tomomi Koshiyama 1, 2, 3, 4, 5 , Masaaki Ohba 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1039/c8dt00161h Yasuhiro Tsuji 1, 2, 3, 4, 5 , Tatsuo Togo 1, 2, 3, 4, 5 , Akio Mishima 1, 2, 3, 4, 5 , Tomomi Koshiyama 1, 2, 3, 4, 5 , Masaaki Ohba 1, 2, 3, 4, 5
Affiliation
|
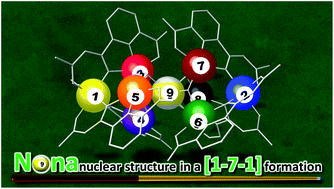
Nonanuclear Ni(II) complexes, [Ni9(Ln)6(OH)6(H2O)6] (Ni9Ln, n = 1–4; H2Ln = 6-acetoacetyl-2-pyridinecarboxylic acid derivatives), were prepared via self-assembly using the asymmetric multidentate ligands H2Ln. A corner-sharing tetrahedron-type structure, [Ni7(μ3-OH)6]8+, and terminal mononuclear units constitute the nonanuclear structure in a [1-7-1] formation. The electrochemical and magnetic properties of Ni9Ln were modulated by the introduction of various substituents in H2Ln.
中文翻译:

源自非对称多齿配体的[1-7-1]结构中的 非核Ni(ii)配合物:磁性和电化学性质†
非核Ni(II)络合物,[Ni 9(L n)6(OH)6(H 2 O)6 ](Ni 9 L n,n = 1-4; H 2 L n = 6-乙酰乙酰基-2-吡啶羧酸酸衍生物),是使用不对称多齿配体H 2 L n通过自组装制备的。甲共角四面体型结构,[镍7(μ 3 -OH)6 ] 8+末端的单核单元以[1-7-1]的形式构成非核结构。通过在H 2 L n中引入各种取代基来调节Ni 9 L n的电化学和磁性。
更新日期:2018-01-29
中文翻译:

源自非对称多齿配体的[1-7-1]结构中的 非核Ni(ii)配合物:磁性和电化学性质†
非核Ni(II)络合物,[Ni 9(L n)6(OH)6(H 2 O)6 ](Ni 9 L n,n = 1-4; H 2 L n = 6-乙酰乙酰基-2-吡啶羧酸酸衍生物),是使用不对称多齿配体H 2 L n通过自组装制备的。甲共角四面体型结构,[镍7(μ 3 -OH)6 ] 8+末端的单核单元以[1-7-1]的形式构成非核结构。通过在H 2 L n中引入各种取代基来调节Ni 9 L n的电化学和磁性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号