当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Fluorescence Quenching by Acylamino Twist in the Excited State for 1-(Acylamino)anthraquinones
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-27 00:00:00 , DOI: 10.1021/acs.jpca.7b11675 Yanliang Zhao 1, 2 , Meishan Wang 1 , Panwang Zhou 2 , Songqiu Yang 2 , Yan Liu 2 , Chuanlu Yang 1 , Yunfan Yang 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-27 00:00:00 , DOI: 10.1021/acs.jpca.7b11675 Yanliang Zhao 1, 2 , Meishan Wang 1 , Panwang Zhou 2 , Songqiu Yang 2 , Yan Liu 2 , Chuanlu Yang 1 , Yunfan Yang 2
Affiliation
|
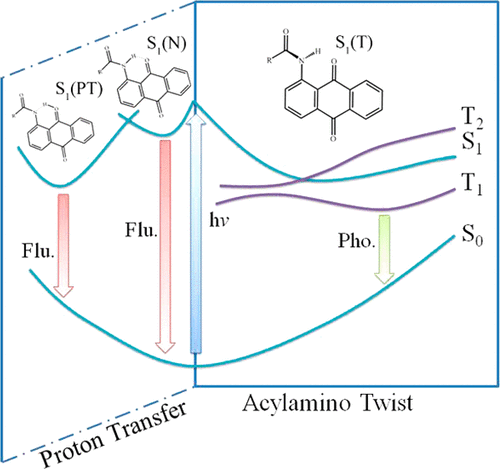
Nitrogen-containing anthraquinone derivatives are widely applied in vegetable fiber dyes. In this paper, the fluorescence quenching mechanism by an acylamino group twist in the excited state for the 1-(acylamino)anthraquinones (AYAAQs) derivatives in acetonitrile is investigated by density functional theory (DFT) and time-dependent density functional theory (TD-DFT) methods. The calculated Stokes shift is in good agreement with the experimental data. The energy profiles show that each AYAAQs derivative reveals a barrierless twist process, indicating that the involvement of acylamino group rotation in addition to proton transfer becomes as another important coordinate in the excited state relaxation pathway. The effects of electron-substituted group promote twist process compared with 1-aminoanthraquinone (AAQ). Then, the cross points are searched by the constructed linearly interpolated internal coordinate (LIIC) pathways for AYAAQs, demonstrating that the potential energy curves of the S1 and T2 states intersect each other and are in accord with the El-Sayed rules. So one can conclude that the acylamino group twist and following intersystem crossing (ISC) processes are important nonradiative inactivation channel for the S1 state of the AYAAQs derivatives, which is more prone to proton transfer process and can explain the low fluorescence efficiency. In addition, we have measured the phosphorescence spectra of AAQ, and on this basis, it can be predicted that the phosphorescence may occur for the AYAAQs derivatives.
中文翻译:

1-(酰基氨基)蒽醌在激发态下通过酰基氨基扭转的荧光猝灭机理。
含氮蒽醌衍生物被广泛应用于植物纤维染料中。本文通过密度泛函理论(DFT)和时变密度泛函理论(TD- DFT)方法。计算得出的斯托克斯位移与实验数据非常吻合。能量图谱表明,每个AYAAQs衍生物都揭示了无障碍扭曲过程,表明除质子转移外,酰氨基基团旋转的参与已成为激发态弛豫途径中的另一个重要坐标。与1-氨基蒽醌(AAQ)相比,电子取代基的作用促进了扭曲过程。然后,1和T 2状态彼此相交,并且符合El-Sayed规则。因此,可以得出结论,对于AYAAQs衍生物的S 1状态,酰氨基基团扭转和随后的系统间交叉(ISC)过程是重要的非辐射灭活通道,它更易于发生质子转移过程,并可以解释低荧光效率。此外,我们已经测量了AAQ的磷光光谱,在此基础上,可以预测AYAAQs衍生物可能发生磷光。
更新日期:2018-01-27
中文翻译:

1-(酰基氨基)蒽醌在激发态下通过酰基氨基扭转的荧光猝灭机理。
含氮蒽醌衍生物被广泛应用于植物纤维染料中。本文通过密度泛函理论(DFT)和时变密度泛函理论(TD- DFT)方法。计算得出的斯托克斯位移与实验数据非常吻合。能量图谱表明,每个AYAAQs衍生物都揭示了无障碍扭曲过程,表明除质子转移外,酰氨基基团旋转的参与已成为激发态弛豫途径中的另一个重要坐标。与1-氨基蒽醌(AAQ)相比,电子取代基的作用促进了扭曲过程。然后,1和T 2状态彼此相交,并且符合El-Sayed规则。因此,可以得出结论,对于AYAAQs衍生物的S 1状态,酰氨基基团扭转和随后的系统间交叉(ISC)过程是重要的非辐射灭活通道,它更易于发生质子转移过程,并可以解释低荧光效率。此外,我们已经测量了AAQ的磷光光谱,在此基础上,可以预测AYAAQs衍生物可能发生磷光。











































 京公网安备 11010802027423号
京公网安备 11010802027423号