当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the enhanced photoelectrochemical performance of hydrothermally controlled hematite nanostructures for proficient solar water oxidation†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1039/c7dt04536k Jin Woo Park 1, 2, 3, 4, 5 , Arunprabaharan Subramanian 1, 2, 3, 4, 5 , Mahadeo A. Mahadik 1, 2, 3, 4, 5 , Su Yong Lee 6, 7, 8, 9 , Sun Hee Choi 6, 7, 8, 9 , Jum Suk Jang 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1039/c7dt04536k Jin Woo Park 1, 2, 3, 4, 5 , Arunprabaharan Subramanian 1, 2, 3, 4, 5 , Mahadeo A. Mahadik 1, 2, 3, 4, 5 , Su Yong Lee 6, 7, 8, 9 , Sun Hee Choi 6, 7, 8, 9 , Jum Suk Jang 1, 2, 3, 4, 5
Affiliation
|
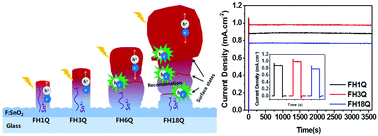
In this paper, we focus on the controlled growth mechanism of α-Fe2O3 nanostructures via the hydrothermal method. The field emission scanning electron microscopy (FESEM) results reveal that at a lower hydrothermal time, the initial nucleation involves the formation of short and thin β-FeOOH nanorods. The subsequent increase in the hydrothermal time leads β-FeOOH to form thicker and longer nanorods. However, high-temperature quenching (HTQ) at 800 °C for 10 min causes the conversion of akaganeite to the hematite phase and activation of hematite by Sn4+ diffusion from a FTO substrate. Sn4+ diffusion from the FTO substrate to the hematite nanostructure was elaborated by X-ray photoelectron spectroscopy (XPS). An α-Fe2O3 nanorod photoanode prepared by a hydrothermal reaction for 3 h and HTQ exhibits the highest photocurrent density of 1.04 mA cm−2. The excellent photoelectrochemical performance could be ascribed to the synergistic effect of the optimum growth of α-Fe2O3 nanorod arrays and Sn4+ diffusion. Intensity modulated photovoltage spectroscopy (IMVS) studies revealed that the α-Fe2O3 photoanodes prepared at 3 h and HTQ exhibited a long electron lifetime (132.69 ms), and contribute to the enhanced PEC performance. The results confirmed that the controlled growth of the β-FeOOH nanorods, as well as Sn4+ diffusion, played a key role in charge transfer during the photoelectrochemical application. The charge transfer mechanisms in α-Fe2O3 nanostructure photoanodes prepared at different hydrothermal times and high-temperature quenching are also investigated.
中文翻译:

深入了解水热控制的赤铁矿纳米结构增强的光电化学性能,可实现高效的太阳能氧化†
在本文中,我们侧重于α-Fe的受控生长机构2 ö 3纳米结构通过水热法。场发射扫描电子显微镜(FESEM)结果表明,在较低的水热时间,初始成核过程涉及形成短而细的β-FeOOH纳米棒。随后水热时间的增加导致β-FeOOH形成更厚,更长的纳米棒。但是,在800°C下进行10分钟的高温淬火(HTQ)会导致赤铁矿转化为赤铁矿相,并通过从FTO基板扩散的Sn 4+引起赤铁矿的活化。锡4+通过X射线光电子能谱(XPS)阐述了从FTO衬底到赤铁矿纳米结构的扩散。一的α-Fe 2 ö 3纳米棒光电阳极制备的水热反应3小时,并表现出HTQ的1.04毫安厘米最高光电流密度-2。优异的光电化学性能可以归因于α-Fe的最佳生长的协同效应2 ö 3纳米棒阵列和Sn 4+扩散。强度调制光电压谱(IMVS)的研究显示,的α-Fe 2 ö 3在3 h和HTQ制备的光阳极具有较长的电子寿命(132.69 ms),并有助于增强PEC性能。结果证实,β-FeOOH纳米棒的受控生长以及Sn 4+的扩散在光电化学应用过程中的电荷转移中起着关键作用。在的α-Fe的电荷转移机构2 ö 3在不同的水热时间和高温淬火制备纳米结构光阳极也进行了研究。
更新日期:2018-01-26
中文翻译:

深入了解水热控制的赤铁矿纳米结构增强的光电化学性能,可实现高效的太阳能氧化†
在本文中,我们侧重于α-Fe的受控生长机构2 ö 3纳米结构通过水热法。场发射扫描电子显微镜(FESEM)结果表明,在较低的水热时间,初始成核过程涉及形成短而细的β-FeOOH纳米棒。随后水热时间的增加导致β-FeOOH形成更厚,更长的纳米棒。但是,在800°C下进行10分钟的高温淬火(HTQ)会导致赤铁矿转化为赤铁矿相,并通过从FTO基板扩散的Sn 4+引起赤铁矿的活化。锡4+通过X射线光电子能谱(XPS)阐述了从FTO衬底到赤铁矿纳米结构的扩散。一的α-Fe 2 ö 3纳米棒光电阳极制备的水热反应3小时,并表现出HTQ的1.04毫安厘米最高光电流密度-2。优异的光电化学性能可以归因于α-Fe的最佳生长的协同效应2 ö 3纳米棒阵列和Sn 4+扩散。强度调制光电压谱(IMVS)的研究显示,的α-Fe 2 ö 3在3 h和HTQ制备的光阳极具有较长的电子寿命(132.69 ms),并有助于增强PEC性能。结果证实,β-FeOOH纳米棒的受控生长以及Sn 4+的扩散在光电化学应用过程中的电荷转移中起着关键作用。在的α-Fe的电荷转移机构2 ö 3在不同的水热时间和高温淬火制备纳米结构光阳极也进行了研究。









































 京公网安备 11010802027423号
京公网安备 11010802027423号