当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methane-to-methanol conversion over zeolite Cu-SSZ-13, and its comparison with the selective catalytic reduction of NOx with NH3†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1039/c7cy02461d Ramon Oord 1, 2, 3, 4, 5 , Joel E. Schmidt 1, 2, 3, 4, 5 , Bert M. Weckhuysen 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1039/c7cy02461d Ramon Oord 1, 2, 3, 4, 5 , Joel E. Schmidt 1, 2, 3, 4, 5 , Bert M. Weckhuysen 1, 2, 3, 4, 5
Affiliation
|
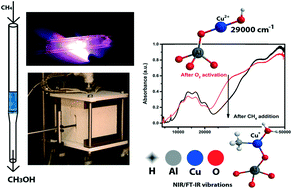
The direct conversion of methane into methanol is considered as one of the holy grails in hydrocarbon chemistry and recently it was found that small pore zeolites, such as Cu-SSZ-13, Cu-SSZ-16 and Cu-SSZ-39, are active for this process. Here, we propose a reaction mechanism based on spectroscopic evidence for the methane-to-methanol reaction over Cu-SSZ-13 (Si/Al = 20). Using in situ FT-IR and operando UV-vis-NIR DRS, performed on a series of different Cu–ion-exchanged SSZ-13 zeolites, both a mono-nuclear site or a dimeric copper active site are consistent with the observations of this study. These proposed active site(s) are characterized by a νOH at ∼3654 cm−1 and a charge transfer (CT) transition at ∼29 000 cm−1. We have further evidence to complete the full catalytic cycle to methanol, including the formation of the reaction intermediate Cu(CH3)(H2O), which is characterized by overtone transitions, i.e., a 2νCH at ∼4200 cm−1 and a 2νOH at ∼5248 cm−1. We found that increasing the pre-oxidation temperature from 450 °C to 550 °C resulted in a 15% increase in methanol production, as well as a concomitant increase of the 29 000 cm−1 CT transition. Furthermore, Cu-exchanged SSZ-13 zeolites, which perform well in the NH3-SCR reaction at 200 °C (the low temperature regime), also show a high activity in the methane-to-methanol reaction and vice versa, leading us to believe that this material has a similar if not the same active site for both the catalytic reduction of NO and the stepwise reaction towards methanol.
中文翻译:

沸石Cu-SSZ-13上的甲烷制甲醇转化及其与NH 3 †选择性催化还原NO x的比较
甲烷直接转化为甲醇被认为是烃化学领域的圣地之一,最近发现小孔沸石(如Cu-SSZ-13,Cu-SSZ-16和Cu-SSZ-39)具有活性对于这个过程。在这里,我们提出基于光谱证据的Cu-SSZ-13(Si / Al = 20)上甲烷制甲醇反应的反应机理。使用原位FT-IR和操作UV-vis-NIR DRS,对一系列不同的Cu-离子交换的SSZ-13沸石进行了分析,单核位点或二聚铜活性位点均与该观察结果一致。学习。这些提出的活性位点(一个或多个)的特征在于ν OH在~3654厘米-1和电荷转移(CT)在过渡〜29000厘米-1。我们进一步证明以完成整个催化循环甲醇中,形成了包括在反应中间体的Cu(CH 3)(H 2 O),其特点是泛音过渡,即,一个2ν CH在~4200厘米-1和2 ν OH在~5248厘米-1。我们发现,将预氧化温度从450°C升高到550°C,可使甲醇产量增加15%,并伴随着29000 cm -1 CT转变的增加。此外,在NH 3中表现良好的铜交换SSZ-13沸石-SCR反应在200°C(低温状态)下,在甲烷转化为甲醇的反应中也显示出很高的活性,反之亦然,这使我们相信这种材料对两种化合物都有相似的活性位点,即使不是相同的活性位点也是如此。催化还原NO和逐步反应生成甲醇。
更新日期:2018-01-26
中文翻译:

沸石Cu-SSZ-13上的甲烷制甲醇转化及其与NH 3 †选择性催化还原NO x的比较
甲烷直接转化为甲醇被认为是烃化学领域的圣地之一,最近发现小孔沸石(如Cu-SSZ-13,Cu-SSZ-16和Cu-SSZ-39)具有活性对于这个过程。在这里,我们提出基于光谱证据的Cu-SSZ-13(Si / Al = 20)上甲烷制甲醇反应的反应机理。使用原位FT-IR和操作UV-vis-NIR DRS,对一系列不同的Cu-离子交换的SSZ-13沸石进行了分析,单核位点或二聚铜活性位点均与该观察结果一致。学习。这些提出的活性位点(一个或多个)的特征在于ν OH在~3654厘米-1和电荷转移(CT)在过渡〜29000厘米-1。我们进一步证明以完成整个催化循环甲醇中,形成了包括在反应中间体的Cu(CH 3)(H 2 O),其特点是泛音过渡,即,一个2ν CH在~4200厘米-1和2 ν OH在~5248厘米-1。我们发现,将预氧化温度从450°C升高到550°C,可使甲醇产量增加15%,并伴随着29000 cm -1 CT转变的增加。此外,在NH 3中表现良好的铜交换SSZ-13沸石-SCR反应在200°C(低温状态)下,在甲烷转化为甲醇的反应中也显示出很高的活性,反之亦然,这使我们相信这种材料对两种化合物都有相似的活性位点,即使不是相同的活性位点也是如此。催化还原NO和逐步反应生成甲醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号