当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple Mechanisms of Zinc-Mediated Inhibition for the Apoptotic Caspases-3, -6, -7, and -8
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1021/acschembio.8b00064 Scott J Eron 1 , Derek J MacPherson 1 , Kevin B Dagbay 1 , Jeanne A Hardy 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1021/acschembio.8b00064 Scott J Eron 1 , Derek J MacPherson 1 , Kevin B Dagbay 1 , Jeanne A Hardy 1
Affiliation
|
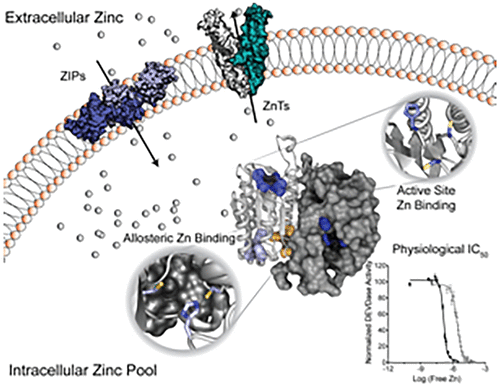
Zinc is emerging as a widely used and important biological regulatory signal. Cellular zinc levels are tightly regulated by a complex array of zinc importers and exporters to control processes such as apoptotic cell death. While caspase inhibition by zinc has been reported previously, the reported inhibition constants were too weak to suggest a critical biological role for zinc-mediated inhibition. In this work, we have adopted a method of assessing available zinc. This allowed assessment of accurate inhibition constants for apoptotic caspases, caspase-3, -6, -7, and -8. Each of these caspases are inhibited by zinc at intracellular levels but with widely differing inhibition constants and different zinc binding stoichiometries. Caspase-3, -6, and -8 appear to be constitutively inhibited by typical zinc levels, and this inhibition must be lifted to allow activation. The inhibition constant for caspase-7 (76 nM) is much weaker than for the other apoptotic caspases (2.6–6.9 nM) suggesting that caspase-7 is not inactivated by normal zinc concentrations but can be inhibited under conditions of zinc stress. Caspase-3, -7, and -8 were found to bind three, one, and two zincs, respectively. In each of these caspases, zinc was present in the active site, in contrast to caspase-6, which binds one zinc allosterically. The most notable new mechanism to emerge from this work is for zinc-mediated inhibition of caspase-8. Zinc binds caspase-8 directly at the active site and at a second site. Zinc binding inhibits formation of the caspase-8 dimer, the activated form of the enzyme. Together these findings suggest that zinc plays a critical role in regulation of apoptosis by direct inactivation of caspases, in a manner that is unique for each caspase.
中文翻译:

锌介导的凋亡 Caspases-3、-6、-7 和 -8 抑制的多种机制
锌正在成为一种广泛使用且重要的生物调节信号。细胞中的锌水平受到一系列复杂的锌输入端和输出端的严格调节,以控制细胞凋亡等过程。虽然之前已经报道过锌对半胱天冬酶的抑制,但所报道的抑制常数太弱,不足以表明锌介导的抑制具有关键的生物学作用。在这项工作中,我们采用了一种评估可用锌的方法。这使得可以准确评估凋亡 caspase、caspase-3、-6、-7 和 -8 的抑制常数。这些半胱天冬酶均在细胞内水平被锌抑制,但抑制常数和锌结合化学计量差异很大。 Caspase-3、-6 和 -8 似乎受到典型锌水平的组成性抑制,并且必须解除这种抑制才能激活。 caspase-7 的抑制常数 (76 nM) 比其他凋亡 caspase (2.6-6.9 nM) 弱得多,表明正常锌浓度不会使 caspase-7 失活,但在锌胁迫条件下会被抑制。 Caspase-3、-7 和 -8 被发现分别结合三个、一个和两个锌。在每一种 caspase 中,锌都存在于活性位点,而 caspase-6 则以变构方式结合一个锌。这项工作中出现的最值得注意的新机制是锌介导的 caspase-8 抑制。锌直接在活性位点和第二个位点结合 caspase-8。锌结合抑制 caspase-8 二聚体(该酶的活化形式)的形成。这些发现共同表明,锌通过直接灭活半胱天冬酶,以每种半胱天冬酶独特的方式,在细胞凋亡调节中发挥关键作用。
更新日期:2018-01-24
中文翻译:

锌介导的凋亡 Caspases-3、-6、-7 和 -8 抑制的多种机制
锌正在成为一种广泛使用且重要的生物调节信号。细胞中的锌水平受到一系列复杂的锌输入端和输出端的严格调节,以控制细胞凋亡等过程。虽然之前已经报道过锌对半胱天冬酶的抑制,但所报道的抑制常数太弱,不足以表明锌介导的抑制具有关键的生物学作用。在这项工作中,我们采用了一种评估可用锌的方法。这使得可以准确评估凋亡 caspase、caspase-3、-6、-7 和 -8 的抑制常数。这些半胱天冬酶均在细胞内水平被锌抑制,但抑制常数和锌结合化学计量差异很大。 Caspase-3、-6 和 -8 似乎受到典型锌水平的组成性抑制,并且必须解除这种抑制才能激活。 caspase-7 的抑制常数 (76 nM) 比其他凋亡 caspase (2.6-6.9 nM) 弱得多,表明正常锌浓度不会使 caspase-7 失活,但在锌胁迫条件下会被抑制。 Caspase-3、-7 和 -8 被发现分别结合三个、一个和两个锌。在每一种 caspase 中,锌都存在于活性位点,而 caspase-6 则以变构方式结合一个锌。这项工作中出现的最值得注意的新机制是锌介导的 caspase-8 抑制。锌直接在活性位点和第二个位点结合 caspase-8。锌结合抑制 caspase-8 二聚体(该酶的活化形式)的形成。这些发现共同表明,锌通过直接灭活半胱天冬酶,以每种半胱天冬酶独特的方式,在细胞凋亡调节中发挥关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号