当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sequential Multicomponent Strategy for the Diastereoselective Synthesis of Densely Functionalized Spirooxindole-Fused Thiazolidines
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acscombsci.7b00179 Giulia Rainoldi 1 , Fabio Begnini 2 , Mariska de Munnik 3 , Leonardo Lo Presti 1 , Christophe M. L. Vande Velde 4 , Romano Orru 3 , Giordano Lesma 1 , Eelco Ruijter 3 , Alessandra Silvani 1
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acscombsci.7b00179 Giulia Rainoldi 1 , Fabio Begnini 2 , Mariska de Munnik 3 , Leonardo Lo Presti 1 , Christophe M. L. Vande Velde 4 , Romano Orru 3 , Giordano Lesma 1 , Eelco Ruijter 3 , Alessandra Silvani 1
Affiliation
|
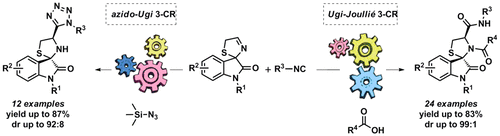
We developed two Ugi-type three-component reactions of spirooxindole-fused 3-thiazolines, isocyanides, and either carboxylic acids or trimethylsilyl azide, to give highly functionalized spirooxindole-fused thiazolidines. Two diverse libraries were generated using practical and robust procedures affording the products in typically good yields. The obtained thiazolidines proved to be suitable substrates for further transformations. Notably, both the Ugi-Joullié and the azido-Ugi reactions resulted highly diastereoselective, affording predominantly the trans-configured products, as confirmed by X-ray crystallographic analysis.
中文翻译:

非对映选择性合成密集官能化螺氧吲哚融合的噻唑烷类化合物的顺序多组分策略
我们开发了螺氧基吲哚稠合的3-噻唑啉,异氰酸酯和羧酸或三甲基甲硅烷基叠氮化物的两个Ugi型三组分反应,以得到高度官能化的螺氧基吲哚稠合的噻唑烷。使用实用且可靠的程序生成了两个不同的库,这些库通常以良好的收率提供产品。所获得的噻唑烷被证明是用于进一步转化的合适底物。值得注意的是,通过X射线晶体学分析证实,Ugi-Joullié反应和azido-Ugi反应均产生非对映选择性,主要提供反式构型产物。
更新日期:2018-01-25
中文翻译:

非对映选择性合成密集官能化螺氧吲哚融合的噻唑烷类化合物的顺序多组分策略
我们开发了螺氧基吲哚稠合的3-噻唑啉,异氰酸酯和羧酸或三甲基甲硅烷基叠氮化物的两个Ugi型三组分反应,以得到高度官能化的螺氧基吲哚稠合的噻唑烷。使用实用且可靠的程序生成了两个不同的库,这些库通常以良好的收率提供产品。所获得的噻唑烷被证明是用于进一步转化的合适底物。值得注意的是,通过X射线晶体学分析证实,Ugi-Joullié反应和azido-Ugi反应均产生非对映选择性,主要提供反式构型产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号