Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor.
Cell ( IF 45.5 ) Pub Date : 2018-Jan-25 , DOI: 10.1016/j.cell.2018.01.006 Matthew R. Janes , Jingchuan Zhang , Lian-Sheng Li , Rasmus Hansen , Ulf Peters , Xin Guo , Yuching Chen , Anjali Babbar , Sarah J. Firdaus , Levan Darjania , Jun Feng , Jeffrey H. Chen , Shuangwei Li , Shisheng Li , Yun O. Long , Carol Thach , Yuan Liu , Ata Zarieh , Tess Ely , Jeff M. Kucharski , Linda V. Kessler , Tao Wu , Ke Yu , Yi Wang , Yvonne Yao , Xiaohu Deng , Patrick P. Zarrinkar , Dirk Brehmer , Dashyant Dhanak , Matthew V. Lorenzi , Dana Hu-Lowe , Matthew P. Patricelli , Pingda Ren , Yi Liu
Cell ( IF 45.5 ) Pub Date : 2018-Jan-25 , DOI: 10.1016/j.cell.2018.01.006 Matthew R. Janes , Jingchuan Zhang , Lian-Sheng Li , Rasmus Hansen , Ulf Peters , Xin Guo , Yuching Chen , Anjali Babbar , Sarah J. Firdaus , Levan Darjania , Jun Feng , Jeffrey H. Chen , Shuangwei Li , Shisheng Li , Yun O. Long , Carol Thach , Yuan Liu , Ata Zarieh , Tess Ely , Jeff M. Kucharski , Linda V. Kessler , Tao Wu , Ke Yu , Yi Wang , Yvonne Yao , Xiaohu Deng , Patrick P. Zarrinkar , Dirk Brehmer , Dashyant Dhanak , Matthew V. Lorenzi , Dana Hu-Lowe , Matthew P. Patricelli , Pingda Ren , Yi Liu
|
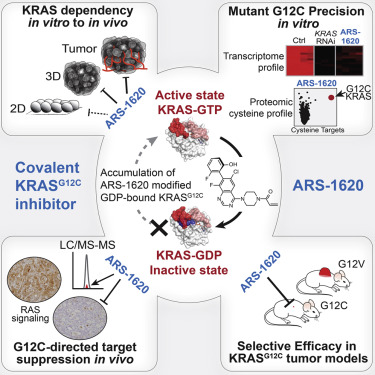
KRASG12C was recently identified to be potentially druggable by allele-specific covalent targeting of Cys-12 in vicinity to an inducible allosteric switch II pocket (S-IIP). Success of this approach requires active cycling of KRASG12C between its active-GTP and inactive-GDP conformations as accessibility of the S-IIP is restricted only to the GDP-bound state. This strategy proved feasible for inhibiting mutant KRAS in vitro; however, it is uncertain whether this approach would translate to in vivo. Here, we describe structure-based design and identification of ARS-1620, a covalent compound with high potency and selectivity for KRASG12C. ARS-1620 achieves rapid and sustained in vivo target occupancy to induce tumor regression. We use ARS-1620 to dissect oncogenic KRAS dependency and demonstrate that monolayer culture formats significantly underestimate KRAS dependency in vivo. This study provides in vivo evidence that mutant KRAS can be selectively targeted and reveals ARS-1620 as representing a new generation of KRASG12C-specific inhibitors with promising therapeutic potential.
中文翻译:

使用共价G12C特异性抑制剂靶向KRAS突变癌。
最近发现,通过等位基因特异性共价靶向Cys-12在可诱导的变构开关II口袋(S-IIP)附近,可以将KRAS G12C药物化。这种方法的成功需要KRAS G12C在其有效GTP和无效GDP构象之间进行主动循环,因为S-IIP的可访问性仅限于GDP约束状态。实践证明,该策略在体外抑制突变型KRAS是可行的。但是,尚不确定该方法是否会转化为体内方法。在这里,我们描述了基于结构的设计和ARS-1620的鉴定,ARS-1620是对KRAS G12C具有高效力和选择性的共价化合物。ARS-1620可实现体内靶标的快速持续维持,从而诱导肿瘤消退。我们使用ARS-1620剖析致癌的KRAS依赖性,并证明单层培养形式大大低估了体内的KRAS依赖性。这项研究提供了体内证据,证明突变KRAS可以被选择性靶向,并揭示了代表新一代KRAS G12C特异性抑制剂的ARS-1620具有广阔的治疗潜力。
更新日期:2018-01-25
中文翻译:

使用共价G12C特异性抑制剂靶向KRAS突变癌。
最近发现,通过等位基因特异性共价靶向Cys-12在可诱导的变构开关II口袋(S-IIP)附近,可以将KRAS G12C药物化。这种方法的成功需要KRAS G12C在其有效GTP和无效GDP构象之间进行主动循环,因为S-IIP的可访问性仅限于GDP约束状态。实践证明,该策略在体外抑制突变型KRAS是可行的。但是,尚不确定该方法是否会转化为体内方法。在这里,我们描述了基于结构的设计和ARS-1620的鉴定,ARS-1620是对KRAS G12C具有高效力和选择性的共价化合物。ARS-1620可实现体内靶标的快速持续维持,从而诱导肿瘤消退。我们使用ARS-1620剖析致癌的KRAS依赖性,并证明单层培养形式大大低估了体内的KRAS依赖性。这项研究提供了体内证据,证明突变KRAS可以被选择性靶向,并揭示了代表新一代KRAS G12C特异性抑制剂的ARS-1620具有广阔的治疗潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号