当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an Efficient Manufacturing Process to GSK2248761A API
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.oprd.7b00357 Celine Bret 1 , Elaine Smith 1 , Erica Vit 1 , Jerome F. Hayes 1 , John D. Hayler 1 , Alan Ironmonger 1 , David Pascoe 1 , Neil Hodnett 1 , Jonathan Stanway 1 , Stephen Etridge 1 , Alistair Stewart 2 , Jingyang Wang 2 , Benjamin A. Mayes 2 , Adel Moussa 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.oprd.7b00357 Celine Bret 1 , Elaine Smith 1 , Erica Vit 1 , Jerome F. Hayes 1 , John D. Hayler 1 , Alan Ironmonger 1 , David Pascoe 1 , Neil Hodnett 1 , Jonathan Stanway 1 , Stephen Etridge 1 , Alistair Stewart 2 , Jingyang Wang 2 , Benjamin A. Mayes 2 , Adel Moussa 2
Affiliation
|
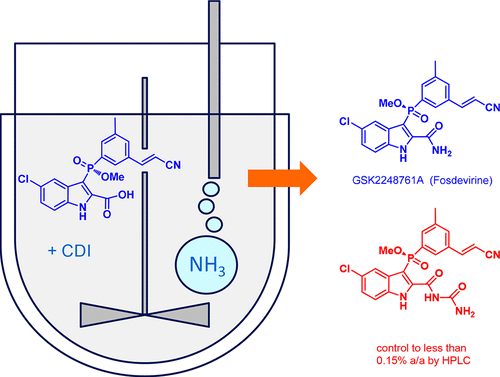
Amidation of indole 2-carboxylate 1 with ammonia gas via the imidazolide 2 gave GSK2248761A API 3, which was in development for the treatment of HIV. Three significant impurities, namely the phosphinic acid 4, the N-acyl urea 8, and the indoloyl carboxamide 6, were formed during the reaction, and the original process was unable to produce API within clinical specification when run at scale. Investigation into the origin, fate, and control of these impurities led to a new process which was able to produce API within clinical specification.
中文翻译:

开发符合GSK2248761A API的高效制造工艺
经由咪唑内酯2用氨气将吲哚2羧酸酯1酰胺化,得到GSK2248761A API 3,该API 3正在开发中,用于治疗HIV。在反应过程中形成了三个重要的杂质,即次膦酸4,N-酰基脲8和吲哚酰羧酰胺6,当大规模运行时,原始工艺无法生产出符合临床规范的API。对这些杂质的来源,命运和控制的研究导致了一种新工艺,该工艺能够生产出符合临床规范的原料药。
更新日期:2018-01-25
中文翻译:

开发符合GSK2248761A API的高效制造工艺
经由咪唑内酯2用氨气将吲哚2羧酸酯1酰胺化,得到GSK2248761A API 3,该API 3正在开发中,用于治疗HIV。在反应过程中形成了三个重要的杂质,即次膦酸4,N-酰基脲8和吲哚酰羧酰胺6,当大规模运行时,原始工艺无法生产出符合临床规范的API。对这些杂质的来源,命运和控制的研究导致了一种新工艺,该工艺能够生产出符合临床规范的原料药。







































 京公网安备 11010802027423号
京公网安备 11010802027423号