当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-Phase Synthesis of β-Amino Ketones Via DNA-Compatible Organocatalytic Mannich Reactions.
ACS Combinatorial Science Pub Date : 2018-01-24 , DOI: 10.1021/acscombsci.7b00151 Nam Tran-Hoang 1 , Thomas Kodadek 1
ACS Combinatorial Science Pub Date : 2018-01-24 , DOI: 10.1021/acscombsci.7b00151 Nam Tran-Hoang 1 , Thomas Kodadek 1
Affiliation
|
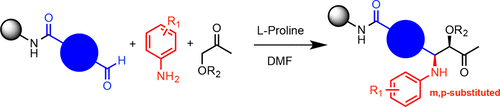
One-bead-one-compound (OBOC) libraries constructed by solid-phase split-and-pool synthesis are a valuable source of protein ligands. Most OBOC libraries are comprised of oligoamides, particularly peptides, peptoids, and peptoid-inspired molecules. Further diversification of the chemical space covered by OBOC libraries is desirable. Toward this end, we report here the efficient proline-catalyzed asymmetric Mannich reaction between immobilized aldehydes and soluble ketones and anilines. The reaction conditions do not compromise the amplification of DNA by the PCR. Thus, this chemistry will likely be useful for the construction of novel DNA-encoded libraries by solid-phase synthesis.
中文翻译:

通过 DNA 相容有机催化曼尼希反应固相合成 β-氨基酮。
通过固相分流合成法构建的一珠一化合物 (OBOC) 文库是蛋白质配体的宝贵来源。大多数 OBOC 文库由寡酰胺组成,特别是肽、类肽和类肽启发的分子。 OBOC 库涵盖的化学空间进一步多样化是可取的。为此,我们在此报告了固定化醛与可溶性酮和苯胺之间有效的脯氨酸催化的不对称曼尼希反应。反应条件不会影响 PCR 的 DNA 扩增。因此,这种化学反应可能有助于通过固相合成构建新型 DNA 编码文库。
更新日期:2018-01-24
中文翻译:

通过 DNA 相容有机催化曼尼希反应固相合成 β-氨基酮。
通过固相分流合成法构建的一珠一化合物 (OBOC) 文库是蛋白质配体的宝贵来源。大多数 OBOC 文库由寡酰胺组成,特别是肽、类肽和类肽启发的分子。 OBOC 库涵盖的化学空间进一步多样化是可取的。为此,我们在此报告了固定化醛与可溶性酮和苯胺之间有效的脯氨酸催化的不对称曼尼希反应。反应条件不会影响 PCR 的 DNA 扩增。因此,这种化学反应可能有助于通过固相合成构建新型 DNA 编码文库。











































 京公网安备 11010802027423号
京公网安备 11010802027423号