当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Anion Identity and Concentration on Electrochemical Reduction of CO2
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-12 , DOI: 10.1002/celc.201701316 Joaquin Resasco 1, 2 , Yanwei Lum 2, 3 , Ezra Clark 1, 2 , Jose Zamora Zeledon 1, 2 , Alexis T. Bell 1, 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-12 , DOI: 10.1002/celc.201701316 Joaquin Resasco 1, 2 , Yanwei Lum 2, 3 , Ezra Clark 1, 2 , Jose Zamora Zeledon 1, 2 , Alexis T. Bell 1, 2
Affiliation
|
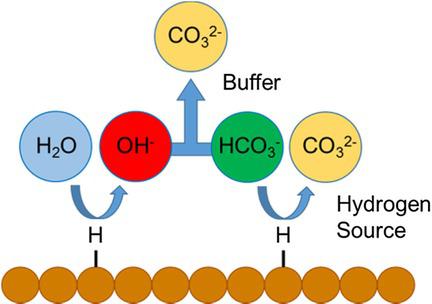
The electrochemical reduction of CO2 is known to be influenced by the concentration and identity of the anionic species in the electrolyte; however, a full understanding of this phenomenon has not been developed. Here, we present the results of experimental and computational studies aimed at understanding the role of electrolyte anions on the reduction of CO2 over Cu surfaces. Experimental studies were performed to show the effects of bicarbonate buffer concentration and the composition of other buffering anions on the partial currents of the major products formed by reduction of CO2 over Cu. It was demonstrated that the composition and concentration of electrolyte anions has relatively little effect on the formation of CO, HCOO−, C2H4, and CH3CH2OH, but has a significant effect on the formation of H2 and CH4. Continuum modeling was used to assess the effects of buffering anions on the pH at the electrode surface. The influence of pH on the activity of Cu for producing H2 and CH4 was also considered. Changes in the pH near the electrode surface were insufficient to explain the differences in activity and selectivity observed with changes in anion buffering capacity observed for the formation of H2 and CH4. Therefore, it is proposed that these differences are the result of the ability of buffering anions to donate hydrogen directly to the electrode surface and in competition with water. The effectiveness of buffering anions to serve as hydrogen donors is found to increase with decreasing pKa of the buffering anion.
中文翻译:

阴离子身份和浓度对电化学还原CO 2的影响
众所周知,CO 2的电化学还原受电解液中阴离子种类的浓度和特性的影响。但是,尚未完全了解这种现象。在这里,我们提出实验和计算研究的结果,旨在了解电解质阴离子对还原Cu表面上的CO 2的作用。进行实验研究以显示碳酸氢盐缓冲液浓度和其他缓冲阴离子的组成对通过CO 2相对于Cu的还原形成的主要产物的分流的影响。已经证实,电解质阴离子的组成和浓度对CO,HCOO的形成中的作用相对较小-,C2 H 4和CH 3 CH 2 OH,但对H 2和CH 4的形成具有显著作用。连续谱模型用于评估缓冲阴离子对电极表面pH值的影响。还考虑了pH对Cu产生H 2和CH 4的活性的影响。电极表面附近的pH值变化不足以解释由于形成H 2和CH 4所观察到的阴离子缓冲能力变化而引起的活性和选择性差异。。因此,提出这些差异是缓冲阴离子将氢直接提供给电极表面并与水竞争的能力的结果。发现缓冲阴离子用作氢供体的有效性随着缓冲阴离子的p K a的降低而增加。
更新日期:2018-02-12
中文翻译:

阴离子身份和浓度对电化学还原CO 2的影响
众所周知,CO 2的电化学还原受电解液中阴离子种类的浓度和特性的影响。但是,尚未完全了解这种现象。在这里,我们提出实验和计算研究的结果,旨在了解电解质阴离子对还原Cu表面上的CO 2的作用。进行实验研究以显示碳酸氢盐缓冲液浓度和其他缓冲阴离子的组成对通过CO 2相对于Cu的还原形成的主要产物的分流的影响。已经证实,电解质阴离子的组成和浓度对CO,HCOO的形成中的作用相对较小-,C2 H 4和CH 3 CH 2 OH,但对H 2和CH 4的形成具有显著作用。连续谱模型用于评估缓冲阴离子对电极表面pH值的影响。还考虑了pH对Cu产生H 2和CH 4的活性的影响。电极表面附近的pH值变化不足以解释由于形成H 2和CH 4所观察到的阴离子缓冲能力变化而引起的活性和选择性差异。。因此,提出这些差异是缓冲阴离子将氢直接提供给电极表面并与水竞争的能力的结果。发现缓冲阴离子用作氢供体的有效性随着缓冲阴离子的p K a的降低而增加。









































 京公网安备 11010802027423号
京公网安备 11010802027423号