当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The nanoscale structure of the electrolyte–metal oxide interface†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1039/c7ee02724a Hans-Georg Steinrück 1, 2, 3, 4 , Chuntian Cao 1, 2, 3, 4, 5 , Yuchi Tsao 4, 6, 7, 8 , Christopher J. Takacs 1, 2, 3, 4 , Oleg Konovalov 9, 10, 11 , Jenel Vatamanu 4, 12, 13, 14, 15 , Oleg Borodin 4, 12, 13, 14, 15 , Michael F. Toney 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1039/c7ee02724a Hans-Georg Steinrück 1, 2, 3, 4 , Chuntian Cao 1, 2, 3, 4, 5 , Yuchi Tsao 4, 6, 7, 8 , Christopher J. Takacs 1, 2, 3, 4 , Oleg Konovalov 9, 10, 11 , Jenel Vatamanu 4, 12, 13, 14, 15 , Oleg Borodin 4, 12, 13, 14, 15 , Michael F. Toney 1, 2, 3, 4
Affiliation
|
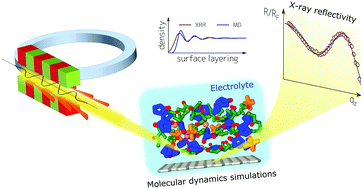
Electrolyte ordering near solid surfaces is of vital importance in diverse fields, ranging from physical chemistry, to energy storage, and heterogeneous catalysis. However, experimental determination of the structure of the electrode–electrolyte interface and electric double layer is challenging due to limited experimental approaches. In this work we show a detailed picture of the electrode–electrolyte interface relevant to Li-ion batteries. Specifically, we probe the atomic-scale interfacial structure of a non-aqueous liquid electrolyte solution of ethylene carbonate (EC) and dimethyl carbonate (DMC) containing lithium hexafluorophosphate (LiPF6) salt via surface sensitive Ångstrom resolution X-ray reflectivity (XRR). We complement our experimental results with molecular dynamics (MD) simulations, and find good agreement between the experiment and simulation derived density profiles. The surface at open circuit voltage (OCV) induces layering of electrolyte molecules near the interface, which decays towards the bulk, and we conclude that both EC and DMC molecules in the first interfacial layer tend to adsorb parallel to the surface. With increasing salt-concentration, the layering periodicity and the degree of order increase. We discuss implications of our results to Li-ion batteries, with focus on the relation between interfacial structure and ion transport in and out of the electrode.
中文翻译:

电解质与金属氧化物界面的纳米级结构†
固体表面附近的电解质有序化在从物理化学到能量存储和多相催化等各个领域都至关重要。然而,由于有限的实验方法,实验确定电极-电解质界面和双电层的结构具有挑战性。在这项工作中,我们显示了与锂离子电池有关的电极-电解质界面的详细图片。具体来说,我们通过六氟磷酸锂(LiPF 6)盐探测了碳酸亚乙酯(EC)和碳酸二甲酯(DMC)的非水电解液的原子尺度界面结构表面敏感埃分辨率X射线反射率(XRR)。我们用分子动力学(MD)模拟来补充实验结果,并在实验与模拟得出的密度分布图之间找到了很好的一致性。开路电压(OCV)下的表面在界面附近引起电解质分子分层,该电解质层向本体衰减,并且我们得出结论,第一界面层中的EC和DMC分子都倾向于平行于表面进行吸附。随着盐浓度的增加,分层的周期性和有序度增加。我们讨论了结果对锂离子电池的影响,重点是界面结构与离子进出电极之间的关系。
更新日期:2018-01-24
中文翻译:

电解质与金属氧化物界面的纳米级结构†
固体表面附近的电解质有序化在从物理化学到能量存储和多相催化等各个领域都至关重要。然而,由于有限的实验方法,实验确定电极-电解质界面和双电层的结构具有挑战性。在这项工作中,我们显示了与锂离子电池有关的电极-电解质界面的详细图片。具体来说,我们通过六氟磷酸锂(LiPF 6)盐探测了碳酸亚乙酯(EC)和碳酸二甲酯(DMC)的非水电解液的原子尺度界面结构表面敏感埃分辨率X射线反射率(XRR)。我们用分子动力学(MD)模拟来补充实验结果,并在实验与模拟得出的密度分布图之间找到了很好的一致性。开路电压(OCV)下的表面在界面附近引起电解质分子分层,该电解质层向本体衰减,并且我们得出结论,第一界面层中的EC和DMC分子都倾向于平行于表面进行吸附。随着盐浓度的增加,分层的周期性和有序度增加。我们讨论了结果对锂离子电池的影响,重点是界面结构与离子进出电极之间的关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号