当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Modulation of Voltage-Gated Sodium Channels by a FGF14-Based Peptidomimetic.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acschemneuro.7b00399 Syed R Ali , Zhiqing Liu , Miroslav N Nenov , Oluwarotimi Folorunso , Aditya Singh , Federico Scala , Haiying Chen , T F James , Musaad Alshammari 1 , Neli I Panova-Elektronova , Mark Andrew White , Jia Zhou , Fernanda Laezza
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acschemneuro.7b00399 Syed R Ali , Zhiqing Liu , Miroslav N Nenov , Oluwarotimi Folorunso , Aditya Singh , Federico Scala , Haiying Chen , T F James , Musaad Alshammari 1 , Neli I Panova-Elektronova , Mark Andrew White , Jia Zhou , Fernanda Laezza
Affiliation
|
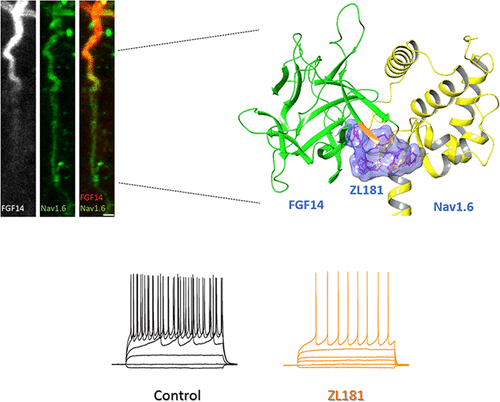
Protein–protein interactions (PPI) offer unexploited opportunities for CNS drug discovery and neurochemical probe development. Here, we present ZL181, a novel peptidomimetic targeting the PPI interface of the voltage-gated Na+ channel Nav1.6 and its regulatory protein fibroblast growth factor 14 (FGF14). ZL181 binds to FGF14 and inhibits its interaction with the Nav1.6 channel C-tail. In HEK-Nav1.6 expressing cells, ZL181 acts synergistically with FGF14 to suppress Nav1.6 current density and to slow kinetics of fast inactivation, but antagonizes FGF14 modulation of steady-state inactivation that is regulated by the N-terminal tail of the protein. In medium spiny neurons in the nucleus accumbens, ZL181 suppresses excitability by a mechanism that is dependent upon expression of FGF14 and is consistent with a state-dependent inhibition of FGF14. Overall, ZL181 and derivatives could lay the ground for developing allosteric modulators of Nav channels that are of interest for a broad range of CNS disorders.
中文翻译:

基于FGF14的拟肽对电压门控钠通道的功能调节。
蛋白质间相互作用(PPI)为CNS药物发现和神经化学探针的开发提供了未开发的机会。在这里,我们介绍ZL181,一种靶向电压门控Na +的PPI接口的新型拟肽Nav1.6通道及其调节蛋白成纤维细胞生长因子14(FGF14)。ZL181与FGF14结合并抑制其与Nav1.6通道C尾的相互作用。在表达HEK-Nav1.6的细胞中,ZL181与FGF14协同发挥作用,以抑制Nav1.6电流密度并减慢快速失活的动力学,但拮抗FGF14稳定状态失活的调节,该失活受蛋白质N末端尾部调节。在伏伏核中的中棘神经元中,ZL181通过一种机制抑制兴奋性,该机制取决于FGF14的表达,并且与FGF14的状态依赖性抑制相一致。总体而言,ZL181及其衍生物可为开发Nav通道的变构调节剂奠定基础,而Nav通道的变构调节剂可引起广泛的CNS疾病。
更新日期:2018-01-23
中文翻译:

基于FGF14的拟肽对电压门控钠通道的功能调节。
蛋白质间相互作用(PPI)为CNS药物发现和神经化学探针的开发提供了未开发的机会。在这里,我们介绍ZL181,一种靶向电压门控Na +的PPI接口的新型拟肽Nav1.6通道及其调节蛋白成纤维细胞生长因子14(FGF14)。ZL181与FGF14结合并抑制其与Nav1.6通道C尾的相互作用。在表达HEK-Nav1.6的细胞中,ZL181与FGF14协同发挥作用,以抑制Nav1.6电流密度并减慢快速失活的动力学,但拮抗FGF14稳定状态失活的调节,该失活受蛋白质N末端尾部调节。在伏伏核中的中棘神经元中,ZL181通过一种机制抑制兴奋性,该机制取决于FGF14的表达,并且与FGF14的状态依赖性抑制相一致。总体而言,ZL181及其衍生物可为开发Nav通道的变构调节剂奠定基础,而Nav通道的变构调节剂可引起广泛的CNS疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号