当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Potent Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors Based on a Phenylimidazole Scaffold.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-01-11 , DOI: 10.1021/acsmedchemlett.7b00488 Michael G Brant 1 , Jake Goodwin-Tindall 1 , Kurt R Stover 1 , Paul M Stafford 1 , Fan Wu 1 , Autumn R Meek 1 , Paolo Schiavini 1 , Stephanie Wohnig 1 , Donald F Weaver 1, 2, 3
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-01-11 , DOI: 10.1021/acsmedchemlett.7b00488 Michael G Brant 1 , Jake Goodwin-Tindall 1 , Kurt R Stover 1 , Paul M Stafford 1 , Fan Wu 1 , Autumn R Meek 1 , Paolo Schiavini 1 , Stephanie Wohnig 1 , Donald F Weaver 1, 2, 3
Affiliation
|
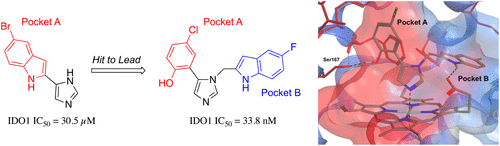
Inhibition of indoleamine 2,3-dioxygenase (IDO1) is an attractive immunotherapeutic approach for the treatment of a variety of cancers. Dysregulation of this enzyme has also been implicated in other disorders including Alzheimer's disease and arthritis. Herein, we report the structure-based design of two related series of molecules: N1-substituted 5-indoleimidazoles and N1-substituted 5-phenylimidazoles. The latter (and more potent) series was accessed through an unexpected rearrangement of an imine intermediate during a Van Leusen imidazole synthesis reaction. Evidence for the binding modes for both inhibitor series is supported by computational and structure-activity relationship studies.
中文翻译:

基于苯基咪唑支架的有效吲哚胺2,3-二加氧酶1(IDO1)抑制剂的鉴定。
吲哚胺2,3-二加氧酶(IDO1)的抑制是一种有吸引力的免疫疗法,用于治疗多种癌症。该酶的失调也与其他疾病有关,包括阿尔茨海默氏病和关节炎。在这里,我们报告了两个相关的一系列分子的基于结构的设计:N1-取代的5-吲哚咪唑和N1-取代的5-苯基咪唑。在Van Leusen咪唑合成反应过程中通过亚胺中间体的意外重排获得了后者(和更有效的)系列。计算和结构-活性关系研究都支持两种抑制剂系列的结合模式的证据。
更新日期:2018-01-22
中文翻译:

基于苯基咪唑支架的有效吲哚胺2,3-二加氧酶1(IDO1)抑制剂的鉴定。
吲哚胺2,3-二加氧酶(IDO1)的抑制是一种有吸引力的免疫疗法,用于治疗多种癌症。该酶的失调也与其他疾病有关,包括阿尔茨海默氏病和关节炎。在这里,我们报告了两个相关的一系列分子的基于结构的设计:N1-取代的5-吲哚咪唑和N1-取代的5-苯基咪唑。在Van Leusen咪唑合成反应过程中通过亚胺中间体的意外重排获得了后者(和更有效的)系列。计算和结构-活性关系研究都支持两种抑制剂系列的结合模式的证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号