当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2‐Iodoxybenzoic Acid Synthesis by Oxidation of 2‐Iodobenzoic Acid at a Boron‐Doped Diamond Anode
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-06 , DOI: 10.1002/celc.201800027 Tomas Bystron 1 , Anastasiia Horbenko 1 , Kamila Syslova 2 , King Kuok Mimi Hii 3 , Klaus Hellgardt 4 , Geoff Kelsall 4
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-06 , DOI: 10.1002/celc.201800027 Tomas Bystron 1 , Anastasiia Horbenko 1 , Kamila Syslova 2 , King Kuok Mimi Hii 3 , Klaus Hellgardt 4 , Geoff Kelsall 4
Affiliation
|
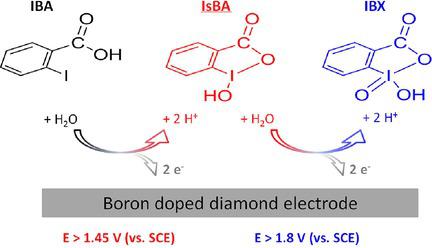
For the first time, the electrochemical synthesis of 2‐iodoxybenzoic acid (IBX), a benign, well‐established, popular and highly selective oxidising agent, is described. The objective of the work was to investigate the possibility of generating IBX electrochemically in aqueous solutions by using boron‐doped diamond anodes. In 0.2 M H2SO4 aqueous solution, 2‐iodobenzoic acid (IBA) was found to be oxidised at potentials >1.6 V vs. SCE, initially to 2‐iodosobenzoic acid (IsBA), which was oxidised to IBX at potentials >1.8 V vs. SCE. Reductions of IBX to IsBA and IsBA to IBA occurred at similar potentials of ca. −0.7 V vs. SCE. The voltammetry results were confirmed by performing a series of batch electrolyses at different electrode potentials. Thus, depending on the electrode potential chosen, IBA can be oxidised anodically either to IsBA or IBX with 100 % overall selectivity. The only side‐reaction was O2 generation, but charge yields did not decrease below 55 % even at conversions >95 %.
中文翻译:

硼掺杂金刚石阳极氧化2-碘代苯甲酸合成2-碘代氧苯甲酸
首次描述了2-碘氧苯甲酸(IBX)的电化学合成,这是一种良性,成熟,受欢迎和高度选择性的氧化剂。这项工作的目的是研究通过使用掺硼金刚石阳极在水溶液中电化学生成IBX的可能性。在0.2 MH 2 SO 4中在水溶液中,发现2-碘代苯甲酸(IBA)在相对于SCE的电位> 1.6 V时被氧化,最初被氧化为2-碘代异苯甲酸(IsBA),然后在相对于SCE的电位> 1.8 V时被氧化为IBX。IBX还原为IsBA和将IsBA还原为IBA的可能性约为ca。−0.7 V相对于SCE。伏安法结果通过在不同电极电势下进行一系列分批电解得到证实。因此,根据所选的电极电势,IBA可以阳极氧化为IsBA或IBX,总选择性为100%。唯一的副反应是O 2生成,但即使转化率> 95%,电荷产率也不会降低到55%以下。
更新日期:2018-02-06
中文翻译:

硼掺杂金刚石阳极氧化2-碘代苯甲酸合成2-碘代氧苯甲酸
首次描述了2-碘氧苯甲酸(IBX)的电化学合成,这是一种良性,成熟,受欢迎和高度选择性的氧化剂。这项工作的目的是研究通过使用掺硼金刚石阳极在水溶液中电化学生成IBX的可能性。在0.2 MH 2 SO 4中在水溶液中,发现2-碘代苯甲酸(IBA)在相对于SCE的电位> 1.6 V时被氧化,最初被氧化为2-碘代异苯甲酸(IsBA),然后在相对于SCE的电位> 1.8 V时被氧化为IBX。IBX还原为IsBA和将IsBA还原为IBA的可能性约为ca。−0.7 V相对于SCE。伏安法结果通过在不同电极电势下进行一系列分批电解得到证实。因此,根据所选的电极电势,IBA可以阳极氧化为IsBA或IBX,总选择性为100%。唯一的副反应是O 2生成,但即使转化率> 95%,电荷产率也不会降低到55%以下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号