当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promotion of Oxygen Reduction with Both Amorphous and Crystalline MnOx through the Surface Engineering of La0.8Sr0.2MnO3‐δ Perovskite
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-12 , DOI: 10.1002/celc.201701248 Jing Yu 1 , Dengjie Chen 2 , Mattia Saccoccio 1 , Kwunyu Lam 1 , Francesco Ciucci 1, 3
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-02-12 , DOI: 10.1002/celc.201701248 Jing Yu 1 , Dengjie Chen 2 , Mattia Saccoccio 1 , Kwunyu Lam 1 , Francesco Ciucci 1, 3
Affiliation
|
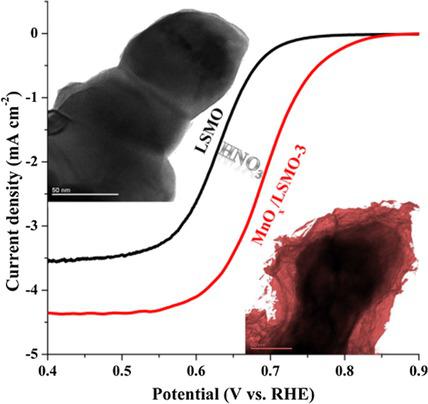
Surfaces play a key role in catalysis. Surfaces can be modified in a number of ways, for example by depositing nanoparticles or with functional coating layers. Here, the surface of La0.8Sr0.2MnO3‐δ (LSMO) is treated with diluted HNO3. This process leads to the preferential formation of MnOx/LSMO on the surface, leading to the exposure of Mn cations at the surface of LSMO. The electrocatalytic activity of the MnOx/LSMO heterostructure towards the oxygen reduction reaction (ORR) is shown to increase when it is compared to the untreated LSMO. Thanks to the formation of MnOx at the surface, the resulting MnOx/LSMO possesses 1) a relatively high specific surface area and a mesoporous structure, 2) a higher coverage of Mn4+/Mn3+ cations at the surface, and 3) a high concentration of highly oxidative oxygen species. This work develops a facile strategy (i. e. HNO3 treatment) for improving the ORR activity of perovskite oxides in alkaline solution at room temperature.
中文翻译:

La0.8Sr0.2MnO3-δ钙钛矿的表面工程促进非晶态和结晶态MnOx的氧还原
表面在催化中起关键作用。可以以多种方式修饰表面,例如通过沉积纳米颗粒或使用功能性涂层。在此,La中的表面0.8的Sr 0.2 MnO的3-δ(LSMO)用稀释的HNO处理3。该过程导致在表面上优先形成MnO x / LSMO,导致Mn阳离子在LSMO表面暴露。与未处理的LSMO相比,MnO x / LSMO异质结构对氧还原反应(ORR)的电催化活性显示出增加的趋势。由于在表面形成了MnO x,因此生成的MnO x/ LSMO具有1)相对较高的比表面积和中孔结构,2)表面上Mn 4+ / Mn 3+阳离子的覆盖率更高,以及3)高浓度的高氧化性氧。这项工作开发了一种简便的策略(即HNO 3处理),用于提高室温下碱性溶液中钙钛矿氧化物的ORR活性。
更新日期:2018-02-12
中文翻译:

La0.8Sr0.2MnO3-δ钙钛矿的表面工程促进非晶态和结晶态MnOx的氧还原
表面在催化中起关键作用。可以以多种方式修饰表面,例如通过沉积纳米颗粒或使用功能性涂层。在此,La中的表面0.8的Sr 0.2 MnO的3-δ(LSMO)用稀释的HNO处理3。该过程导致在表面上优先形成MnO x / LSMO,导致Mn阳离子在LSMO表面暴露。与未处理的LSMO相比,MnO x / LSMO异质结构对氧还原反应(ORR)的电催化活性显示出增加的趋势。由于在表面形成了MnO x,因此生成的MnO x/ LSMO具有1)相对较高的比表面积和中孔结构,2)表面上Mn 4+ / Mn 3+阳离子的覆盖率更高,以及3)高浓度的高氧化性氧。这项工作开发了一种简便的策略(即HNO 3处理),用于提高室温下碱性溶液中钙钛矿氧化物的ORR活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号