当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of Backbone Pattern and Residue Substitution on Helicity in α/β/γ-Peptides
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b10868 Young-Hee Shin 1 , Samuel H. Gellman 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b10868 Young-Hee Shin 1 , Samuel H. Gellman 1
Affiliation
|
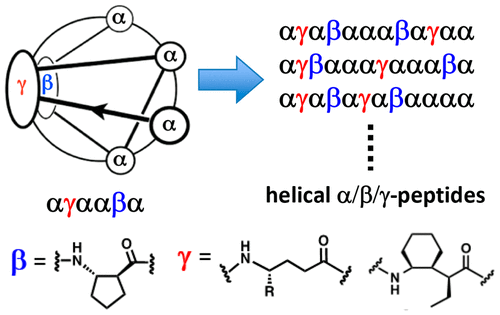
We have evaluated the impact of changes in the chemical structure of peptidic oligomers containing α-, β-, and γ-amino acid residues (α/β/γ-peptides) on the propensities of these oligomers to adopt helical conformations in aqueous and alcoholic solutions. These studies were inspired by our previous discovery that α/β/γ-peptides containing a regular αγααβα hexad repeat adopt an α-helix-like conformation in which the β and γ residues are aligned in a stripe along one side, and the remainder of the helix surface is defined by the α residues. This helix was found to be most stable when the β and γ residues were rigidified with specific cyclic constraints. Relaxation of the β residue constraints caused profound conformational destabilization, but relaxation of the γ residue constraints led to only a moderate drop in helicity. The new work more broadly characterizes the effect of γ residue substitution on helix stability, based on circular dichroism and two-dimensional NMR measurements. We find that even a fully unsubstituted γ residue (derived from γ-aminobutyric acid) supports a moderate helical propensity, which is surprising in light of the strong destabilizing effect of glycine residues on α-helix stability. Additional studies examine the effects of altering sequence in terms of amino acid type, by comparing a prototype with the αγααβα hexad pattern to isomers with irregular arrangements of the α, β, and γ residues along the backbone. The data indicate that the strong helix-forming propensity previously discovered for α/β/γ-peptide 12-mers is retained when sequence is varied, with small variations detected across diverse α-β-γ placements. These structural findings suggest that α/β/γ-peptide scaffolds represent versatile scaffolds for the design of peptidic foldamers that display specific functions.
中文翻译:

骨架模式和残基取代对α/β/γ-肽中螺旋度的影响
我们评估了含有 α-、β- 和 γ-氨基酸残基(α/β/γ-肽)的肽寡聚体化学结构变化对这些寡聚体在水性和醇性中采用螺旋构象的倾向的影响。解决方案。这些研究的灵感来自我们之前的发现,即含有规则 αγααβα 六联体重复序列的 α/β/γ-肽采用 α-螺旋状构象,其中 β 和 γ 残基沿一侧排列成条状,其余部分螺旋表面由 α 残基定义。当 β 和 γ 残基被特定的循环约束刚性化时,发现该螺旋最稳定。β 残基约束的放松导致严重的构象不稳定,但 γ 残基约束的放松仅导致螺旋度的适度下降。这项新工作基于圆二色性和二维 NMR 测量,更广泛地表征了 γ 残基取代对螺旋稳定性的影响。我们发现,即使是完全未取代的 γ 残基(源自 γ-氨基丁酸)也支持适度的螺旋倾向,鉴于甘氨酸残基对 α-螺旋稳定性的强烈不稳定影响,这是令人惊讶的。其他研究通过将具有 αγααβα 六联体模式的原型与沿骨架具有不规则排列的 α、β 和 γ 残基的异构体进行比较,来检查改变序列在氨基酸类型方面的影响。数据表明,当序列变化时,先前发现的 α/β/γ-肽 12 聚体的强螺旋形成倾向得以保留,在不同的 α-β-γ 位置中检测到小的变化。
更新日期:2018-01-19
中文翻译:

骨架模式和残基取代对α/β/γ-肽中螺旋度的影响
我们评估了含有 α-、β- 和 γ-氨基酸残基(α/β/γ-肽)的肽寡聚体化学结构变化对这些寡聚体在水性和醇性中采用螺旋构象的倾向的影响。解决方案。这些研究的灵感来自我们之前的发现,即含有规则 αγααβα 六联体重复序列的 α/β/γ-肽采用 α-螺旋状构象,其中 β 和 γ 残基沿一侧排列成条状,其余部分螺旋表面由 α 残基定义。当 β 和 γ 残基被特定的循环约束刚性化时,发现该螺旋最稳定。β 残基约束的放松导致严重的构象不稳定,但 γ 残基约束的放松仅导致螺旋度的适度下降。这项新工作基于圆二色性和二维 NMR 测量,更广泛地表征了 γ 残基取代对螺旋稳定性的影响。我们发现,即使是完全未取代的 γ 残基(源自 γ-氨基丁酸)也支持适度的螺旋倾向,鉴于甘氨酸残基对 α-螺旋稳定性的强烈不稳定影响,这是令人惊讶的。其他研究通过将具有 αγααβα 六联体模式的原型与沿骨架具有不规则排列的 α、β 和 γ 残基的异构体进行比较,来检查改变序列在氨基酸类型方面的影响。数据表明,当序列变化时,先前发现的 α/β/γ-肽 12 聚体的强螺旋形成倾向得以保留,在不同的 α-β-γ 位置中检测到小的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号