当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
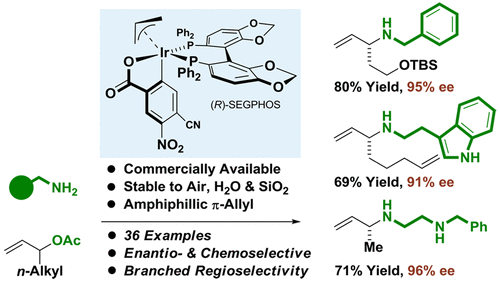
Amphiphilic π-Allyliridium C,O-Benzoates Enable Regio- and Enantioselective Amination of Branched Allylic Acetates Bearing Linear Alkyl Groups
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b13482 Arismel Tena Meza 1 , Thomas Wurm 2 , Lewis Smith 1 , Seung Wook Kim 2 , Jason R. Zbieg 1 , Craig E. Stivala 1 , Michael J. Krische 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b13482 Arismel Tena Meza 1 , Thomas Wurm 2 , Lewis Smith 1 , Seung Wook Kim 2 , Jason R. Zbieg 1 , Craig E. Stivala 1 , Michael J. Krische 2
Affiliation
|
The first examples of amphiphilic reactivity in the context of enantioselective catalysis are described. Commercially available π-allyliridium C,O-benzoates, which are stable to air, water and SiO2 chromatography, and are well-known to catalyze allyl acetate-mediated carbonyl allylation, are now shown to catalyze highly chemo-, regio- and enantioselective substitutions of branched allylic acetates bearing linear alkyl groups with primary amines.
中文翻译:

两亲性 π-烯丙基铱 C,O-苯甲酸酯使带有直链烷基的支化烯丙基乙酸酯的区域和对映选择性胺化成为可能
描述了对映选择性催化背景下两亲反应性的第一个例子。市售的 π-烯丙基 C,O-苯甲酸酯对空气、水和 SiO2 色谱稳定,众所周知可催化乙酸烯丙酯介导的羰基烯丙基化,现在显示可催化高度化学、区域和对映选择性取代带有直链烷基和伯胺的支链乙酸烯丙酯。
更新日期:2018-01-19
中文翻译:

两亲性 π-烯丙基铱 C,O-苯甲酸酯使带有直链烷基的支化烯丙基乙酸酯的区域和对映选择性胺化成为可能
描述了对映选择性催化背景下两亲反应性的第一个例子。市售的 π-烯丙基 C,O-苯甲酸酯对空气、水和 SiO2 色谱稳定,众所周知可催化乙酸烯丙酯介导的羰基烯丙基化,现在显示可催化高度化学、区域和对映选择性取代带有直链烷基和伯胺的支链乙酸烯丙酯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号