Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of pH and temperature on the electrochemical and semiconducting properties of zinc in alkaline buffer media
RSC Advances ( IF 3.9 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1039/c7ra12723e F El-Taib Heakal 1 , W R Abd-Ellatif 2 , N S Tantawy 2 , A A Taha 2
RSC Advances ( IF 3.9 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1039/c7ra12723e F El-Taib Heakal 1 , W R Abd-Ellatif 2 , N S Tantawy 2 , A A Taha 2
Affiliation
|
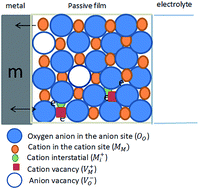
The electrochemical and semiconductive properties of spontaneously formed passive films on pure Zn were investigated in alkaline carbonate/bicarbonate buffer solutions as functions of pH and temperature. The study was performed in 0.1 M (CO32− + HCO3−) mixtures over the pH range 9.2 to 9.8 using open circuit potential, electrochemical impedance spectroscopy (EIS), potentiodynamic polarization and Mott–Schottky analysis techniques. Generally, zinc passivation is enhanced with either increasing pH or decreasing the ambient temperature. The steady state potential (Ess) value reveals that in pH 9.8 buffer the propensity of Zn for passivation is superior when compared with those in the other tested buffer solutions. The total surface film resistance (Rt) derived from the impedance data proves this result, which is likely attributed to changes in composition and/or microstructure of the film. In pH 9.8 buffer solution the passivation tendency always decreases with temperature increase. However, in pH 9.2 the system behaves similarly up to 25 °C; afterwards zinc passivation trend was found to re-increase once more. The apparent activation energy for the corrosion process was evaluated and discussed. Analysis of Mott–Schottky plots was found to be suitable for characterizing the semiconductor properties of the naturally deposited barrier layers which are all consistent with the well-known n-type character of the oxide film on zinc. The absence of any evidences for the p-type semiconductive behavior indicates a preponderance of oxygen vacancies and zinc interstitials over metal vacancies. Moreover, Mott–Schottky results demonstrate that the donor concentration increases with either increasing pH or deceasing temperature commensurate with the increasing trends in the passive film thickness.
中文翻译:

pH和温度对碱性缓冲介质中锌的电化学和半导体性能的影响
在碱性碳酸盐/碳酸氢盐缓冲溶液中研究了纯锌上自发形成的钝化膜的电化学和半导体特性,作为 pH 和温度的函数。该研究使用开路电位、电化学阻抗谱 (EIS)、动电位极化和 Mott-Schottky 分析技术在 0.1 M (CO 3 2- + HCO 3 - ) 混合物中在 9.2 至 9.8 的 pH 范围内进行。通常,锌钝化随着 pH 值的增加或环境温度的降低而增强。稳态电位 ( E ss) 值表明,与其他测试的缓冲溶液相比,在 pH 9.8 缓冲液中,Zn 的钝化倾向优于其他缓冲溶液。总表面膜电阻(R t) 从阻抗数据得出的结果证明了这一结果,这可能归因于薄膜的成分和/或微观结构的变化。在 pH 9.8 的缓冲溶液中,钝化趋势总是随着温度的升高而降低。然而,在 pH 值为 9.2 时,系统在高达 25 °C 时表现类似;之后发现锌钝化趋势再次增加。对腐蚀过程的表观活化能进行了评估和讨论。发现莫特-肖特基图的分析适用于表征自然沉积阻挡层的半导体特性,这些特性都与众所周知的锌上氧化膜的 n 型特性一致。没有任何关于 p 型半导体行为的证据表明氧空位和锌间隙比金属空位占优势。而且,
更新日期:2018-01-19
中文翻译:

pH和温度对碱性缓冲介质中锌的电化学和半导体性能的影响
在碱性碳酸盐/碳酸氢盐缓冲溶液中研究了纯锌上自发形成的钝化膜的电化学和半导体特性,作为 pH 和温度的函数。该研究使用开路电位、电化学阻抗谱 (EIS)、动电位极化和 Mott-Schottky 分析技术在 0.1 M (CO 3 2- + HCO 3 - ) 混合物中在 9.2 至 9.8 的 pH 范围内进行。通常,锌钝化随着 pH 值的增加或环境温度的降低而增强。稳态电位 ( E ss) 值表明,与其他测试的缓冲溶液相比,在 pH 9.8 缓冲液中,Zn 的钝化倾向优于其他缓冲溶液。总表面膜电阻(R t) 从阻抗数据得出的结果证明了这一结果,这可能归因于薄膜的成分和/或微观结构的变化。在 pH 9.8 的缓冲溶液中,钝化趋势总是随着温度的升高而降低。然而,在 pH 值为 9.2 时,系统在高达 25 °C 时表现类似;之后发现锌钝化趋势再次增加。对腐蚀过程的表观活化能进行了评估和讨论。发现莫特-肖特基图的分析适用于表征自然沉积阻挡层的半导体特性,这些特性都与众所周知的锌上氧化膜的 n 型特性一致。没有任何关于 p 型半导体行为的证据表明氧空位和锌间隙比金属空位占优势。而且,











































 京公网安备 11010802027423号
京公网安备 11010802027423号